Question
Question: D-Glucose and D-Mannose are the example of. A) \(C - 4epimer\) B) \(C - 3epimer\) C) \(C - 2e...
D-Glucose and D-Mannose are the example of.
A) C−4epimer
B) C−3epimer
C) C−2epimer
D) C−2anomer
Solution
We know that Compounds having the same atoms or groups with different spatial arrangements are called stereoisomers. There are two types of Stereoisomers; they are (1) Geometrical isomer and (2) optical isomer.
Complete step by step answer:
At a stereogenic centre, two isomers present in the molecules differ, while the rest remains the same. A molecule may contain many stereocenters leading to numerous stereocenters.
Now we can draw the structure of D-glucose and D-mannose as,
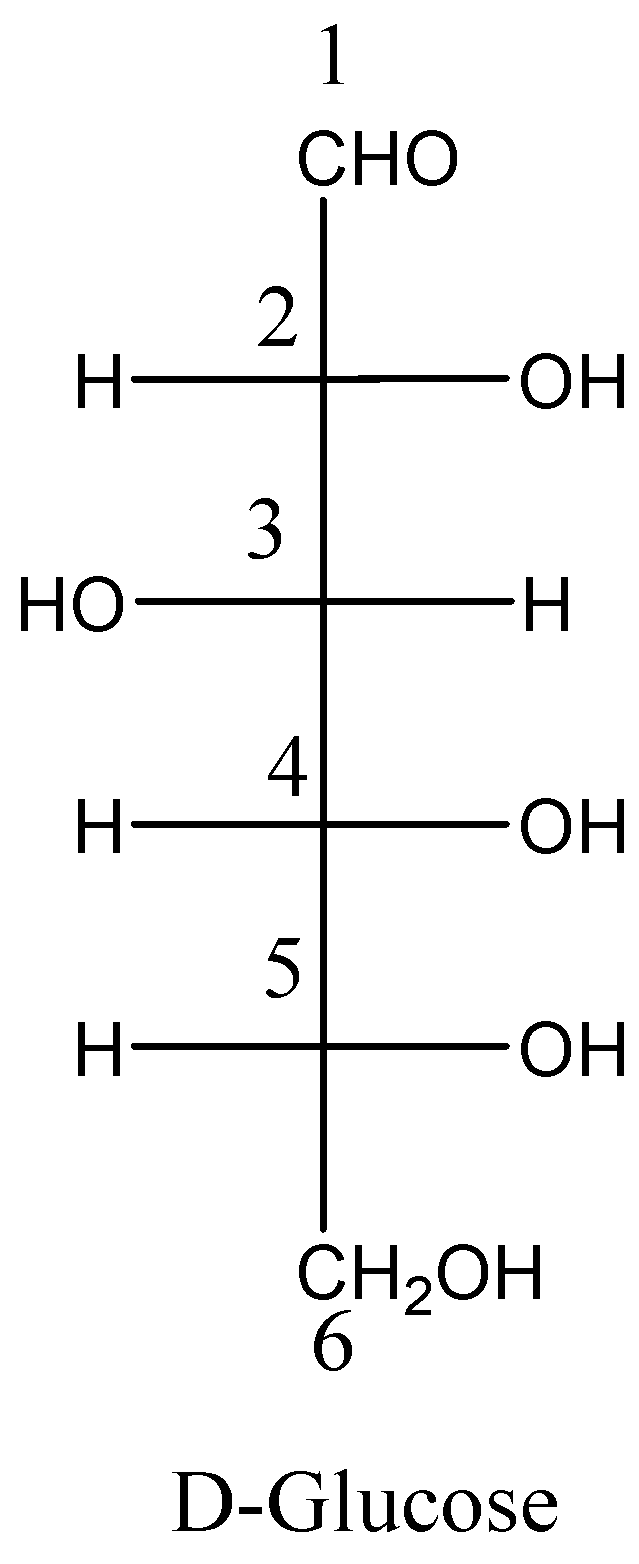
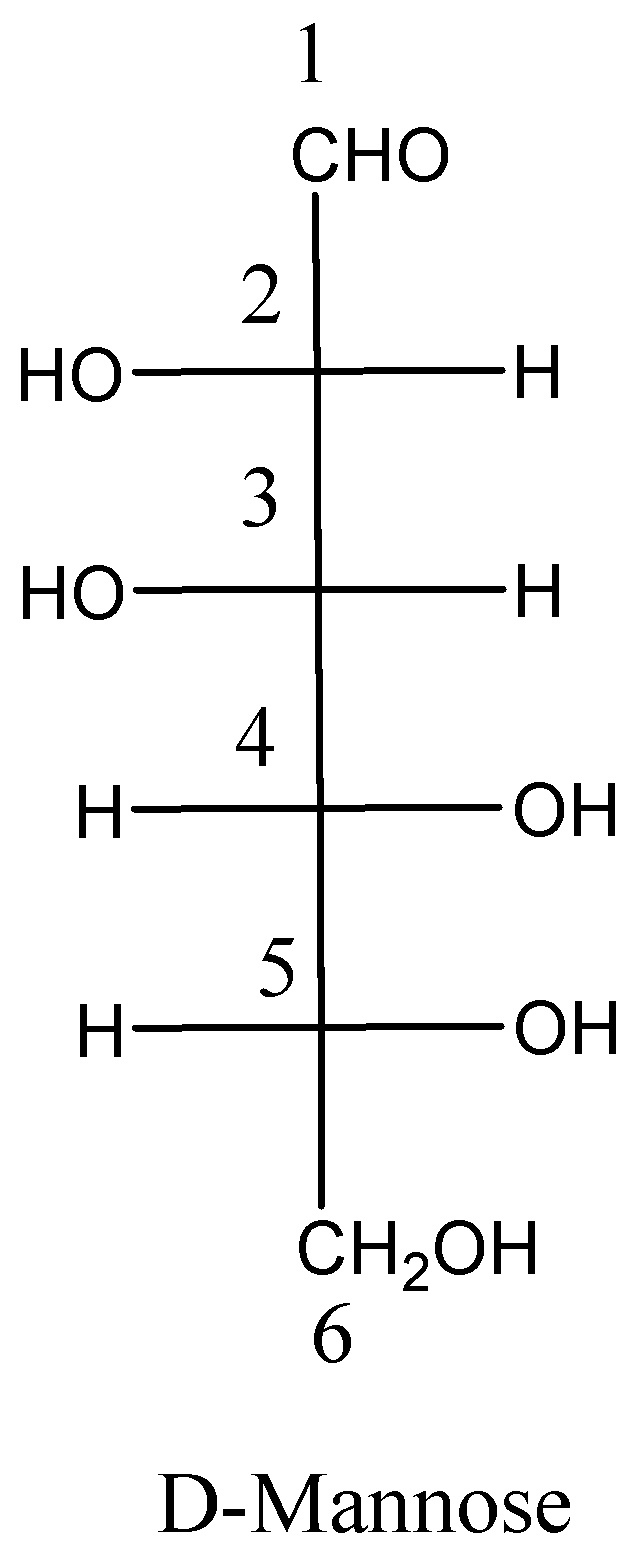
We must remember that the epimer are diastereoisomers that have the alternative configuration at just one of 2 or a lot of chiral centers within the individual molecular entities. D-Mannose is an epimer of D-glucose because the two sugars vary only in the configuration at C−2.
Therefore, the option C is correct.
As we know that the two sugars are having the same configuration at C−3 and C−4 positions. Therefore, the options A and B are incorrect.
Anomers:
We also remember that the anomerism is unique for single bonded ring structures where "cis" or "E" and "trans" or "Z" wants to name the substitutions on a carbon atom that also exhibit the uniqueness of chirality; so Anomers have carbon atoms that have geometric isomerism and optical isomerism on one or more of the carbons of the ring.
Note:
Now we can see how enantiomers and diastereomers differ from each other.
Let we see the details about enantiomers as,
-Enantiomers: An object or a molecule which cannot be superimposed on its mirror image must be asymmetric. Such a pair of molecules related to each other as an object to its mirror image is known as enantiomorphs or enantiomers. The terms chiral and achiral are used to designate dissymmetric and non- dissymmetric molecules respectively. A chiral molecule is not superimposable on its likeness whereas achiral molecule is superimposable on its mirror image. A chiral atom is any atom barren of plane of symmetry and is additionally referred to as chiral centre. Enantiomers exist solely just in case of chiral molecules.
Let we discuss about the details of diastereomers
-Diastereomers: Diastereomers are stereoisomers not connected through a mirrored image process. They’re not mirror pictures of every other. These contain meso compounds, cis–trans isomers, E-Z isomers, and non-enantiomeric optical isomers. Diastereomers seldom have similar physical properties. For instance, the meso variety of hydroxy acid types a diastereomeric try with each levo and Dextro salt acids, which form an enantiomeric pair.
