Question
Question: ...
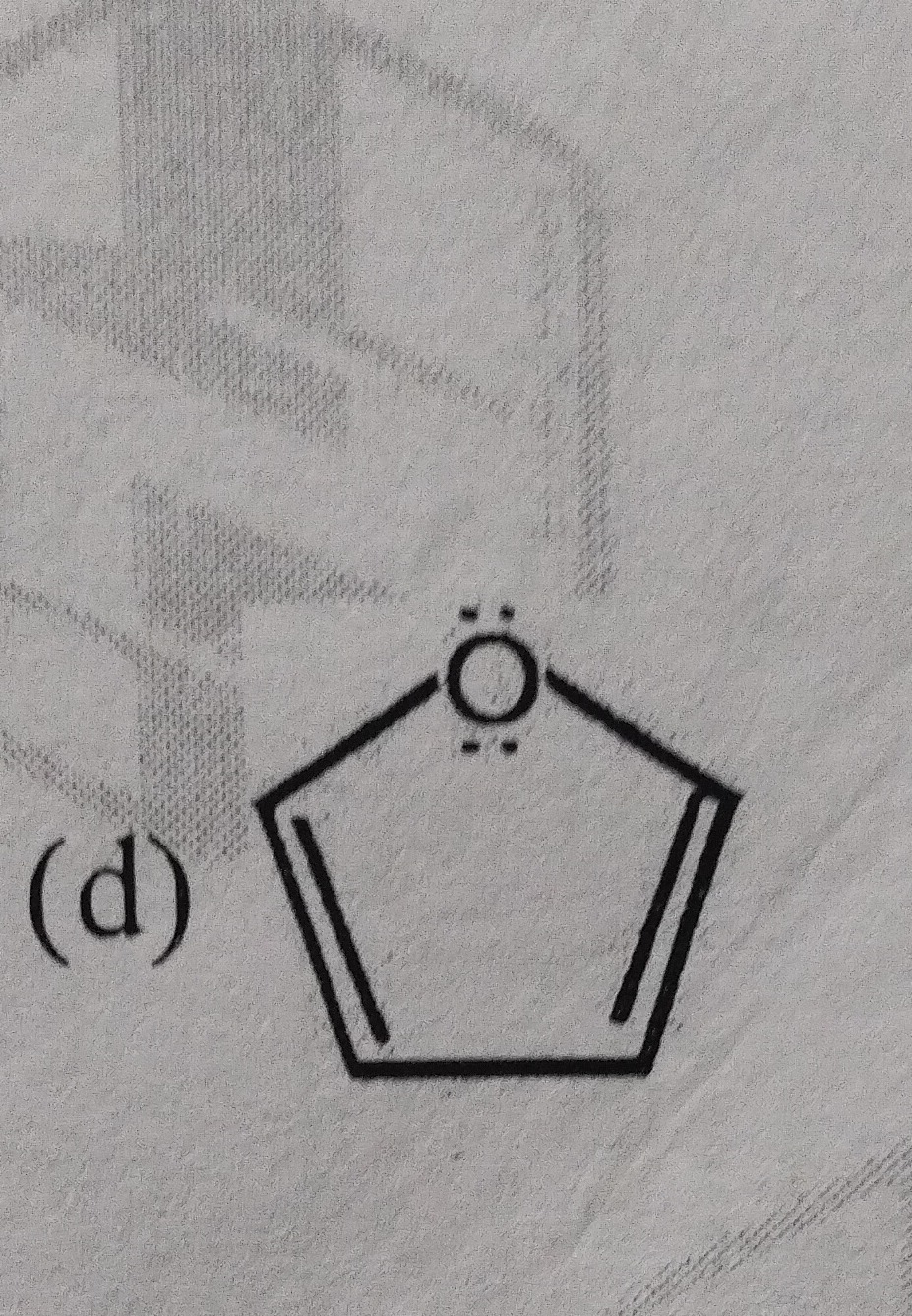
Answer
The compound shown in the figure is Furan.
Explanation
Solution
The provided structure is a five-membered cyclic ether with two double bonds. This compound is known as Furan. It is an aromatic compound because it is cyclic, planar, fully conjugated, and contains 6 pi electrons (4 from the two double bonds and 2 from one lone pair on the oxygen atom), satisfying Hückel's (4n+2) rule for n=1.
- Ring Structure: It is a cyclic compound forming a five-membered ring.
- Heteroatom: The ring contains one oxygen atom, making it a heterocyclic compound.
- Unsaturation: There are two double bonds within the ring.
- Aromaticity:
- The molecule is cyclic and planar.
- It has 2 double bonds, contributing 2×2=4 pi electrons.
- The oxygen atom has two lone pairs. One of these lone pairs is in a p-orbital and participates in the resonance, contributing 2 pi electrons to the system, while the other lone pair is in an sp² orbital and does not participate in aromaticity.
- Total pi electrons = 4 (from double bonds) + 2 (from one lone pair of oxygen) = 6 pi electrons.
- According to Hückel's rule, a cyclic, planar, fully conjugated system with (4n + 2) pi electrons is aromatic. For n=1, (4*1 + 2) = 6 pi electrons.
- Since the molecule has 6 pi electrons and meets the other criteria, it is an aromatic compound.
