Question
Question: D-Erythrose and D-threose is separately reduced with \(NaB{{H}_{4}}\) to give the products (A) and (...
D-Erythrose and D-threose is separately reduced with NaBH4 to give the products (A) and (B) respectively. Which of the following statements is correct about (A) and (B)?
A. Both (A) and (B) will be optically active
B. Both (A) and (B) will be optically inactive
C. (A) will be optically inactive but (B) will be optically active
D. Neither (A) or (B) possesses any asymmetric carbon atom.
Solution
To find the correct option you have to draw the structure of D-Erythrose and D-threose and then treat it with the NaBH4 and check whether the mirror image of products A and B is superimposed/non-superimposable and symmetrical/asymmetrical. If the mirror image will be superimposable and symmetrical then it will be optically inactive and vice versa.
Complete step by step answer:
From your chemistry lessons you have learned about the properties and characteristics of optically active and optically inactive compounds.
Optically active compounds are those compounds which rotate the place of polarized light and when this compound is placed in front of the mirror then their mirror images are asymmetrical i.e. cannot be broken from the centre and they are non-superimposable on each other.
Whereas optical inactive compounds are those compounds which are not able to rotate the plane of polarized light and the mirror image of these compounds are symmetrical and superimposable.
Now in the question we have to check the mirror image of the products formed when D-Erythrose and D-threose are reduced separately NaBH4. So, the products of D-Erythrose and D-threose after reducing with NaBH4 is:

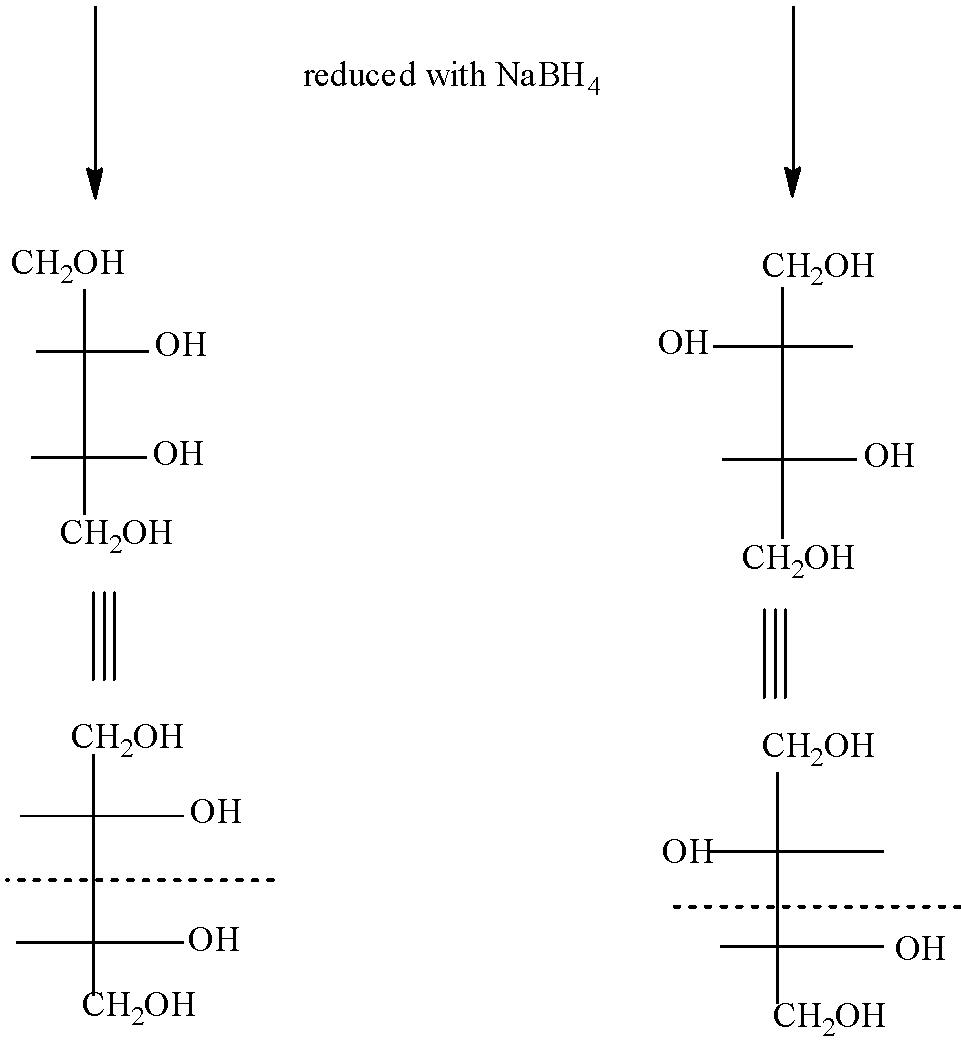
As you can see the mirror image of the (A) is superimposable and symmetrical therefore it would be optically inactive and the mirror image of (B) is non-superimposable and asymmetrical with each other, hence it is optically active.
So, the correct answer is “Option C”.
Note: Optical activity always occurs only in chiral compounds. All chiral compounds are optically active and optically active substances are stable. Chiral compounds or molecules are those in which a central atom is surrounded by four other atoms and chiral compounds are non-superimposable with their mirror image. Opposite of chiral is achiral.
