Question
Question: Cyclopentadienyl anion is: A.Benzenoid and aromatic B.Non-benzenoid and aromatic C.Non-benzeno...
Cyclopentadienyl anion is:
A.Benzenoid and aromatic
B.Non-benzenoid and aromatic
C.Non-benzenoid and non-aromatic
D.Non-benzenoid and anti-aromatic
Solution
A compound should have a benzene ring so as to be a benzenoid aromatic compound. An aromatic compound follows Huckel’s Rule which states that a compound must have 4n+2 pi-electrons to attain aromaticity. A compound can be aromatic and non-benzenoid at the same time if it follows huckel’s rule but does not have benzene structure.
Complete step-by-step answer:
To be an aromatic compound or to attain aromaticity, a compound must fulfil these four conditions:
1.The molecule should be cyclic.
2.Every atom in the cyclic ring must be conjugated which provides the cyclic ring delocalized pi-electron system.
3.The compound must follow Huckel’s rule i.e. 4n+2 pi-electrons.
4.The molecule must be flat or planar as they possess large potential energy.
According to Huckel’s rule, all planar and aromatic compounds must have [4n+2] pi-electrons where n is an integer where n = 0, 1, 2, etc. Cyclopentadienyl anion has a planar ring structure with two pi bonds and four pi-electrons. The anion contributes two more electrons to the system which are in conjugation with the two double bonds and makes it a six-electron system. This now follows Huckel’s rule i.e. [4n+2] rule in which n=1 . Thus, it is an aromatic compound.
Benzenoid compounds are those which have benzene or benzene-like structure. If we look at the structure of cyclopentadienyl anion, we can easily see that it does not have benzene like structure because benzene has six carbon atoms but it has only five carbon atoms. So, it is not benzenoid and we can call it non-benzenoid structure.
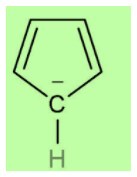
Hence the correct option is (B).
Note: If we look at cyclopentadienyl cation, we can see that it has only two double bonds in conjugation i.e. 4 pi-electrons only. As it does not follow Huckel’s rule ( 4n+2 pi-electrons), it is called as anti-aromatic compound. Anti-aromatic compounds are cyclic, conjugated and have 4n pi-electrons and are flat too.
