Question
Question: Cyclopentadienyl anion is: A. Benzenoid and aromatic B. Non-benzenoid and aromatic C. Non-benz...
Cyclopentadienyl anion is:
A. Benzenoid and aromatic
B. Non-benzenoid and aromatic
C. Non-benzenoid and non-aromatic
D. Non-benzenoid and anti-aromatic
Solution
Cyclopentadienyl is present in a form of free radical in the shape of five membered ring and have the molecular formula (4n+2)π C5H5and cyclopentadienyl anion is shown by the molecular formula C5H5−and abbreviated as Cp−.
Complete answer:
To be an aromatic compound, a compound must follow some conditions:
1. It should be cyclic in nature and the compound given contains a 5-membered cyclic ring.
2. Every atom in the ring must be conjugated which delocalize the pi electron system.
3. The compound must follow Huckel’s rule i.e. (4n+2)πelectrons i.e. the total number of electrons present should follows this rule and n can be any integer i.e. 1,2, 3….. but always have a whole number.
In the given compound anion contributes two more electrons to the compound and 4 pi electrons are already so we can say that it follows Huckel’s rule with 6πelectrons where n is 1.
4. Molecules must be planar as we know that planar molecules possess higher potential energy.
Given compound follows all the properties of being aromatic, so it is aromatic in nature on the other hand the compound is said to be benzenoid if it contains benzene ring but it contains a five-membered ring so non-benzenoid in nature. This can be shown as follows:
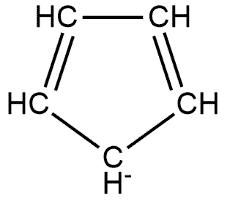
Hence option B is the correct answer.
Note:
Cyclopentadienyl anion is formed by the deprotonation of the molecule cyclopentadienyl. On the other hand if we take the case of cyclopentadienyl cation then it does not follow Huckel’s rule as there are only 4πelectrons present so it becomes anti-aromatic in nature.
