Question
Question: Cyclohexanone on being treated with NaOH solution forms: A.
B.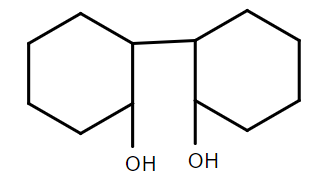
C.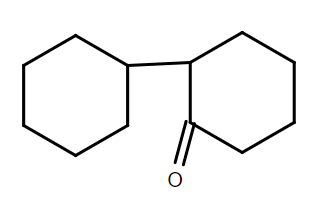
D.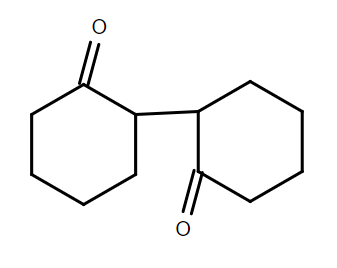
Solution
To answer this question, you should recall the concept of an aldol condensation. The reaction of aldehydes and ketones containing at least one hydrogen is treated with dilute alkali; they form -hydroxy aldehydes or -hydroxy ketones (ketol) respectively.
Complete step by step answer:
We know that in aldol condensation the hydroxide ion functions as a base moving the acidic hydrogen-producing the reactive enolate ion. Further, the aldehyde is attacked at the electrophilic carbonyl carbon by the nucleophilic enolate ion resulting in an alkoxide intermediate. The alkoxide ion now formed deprotonates the water molecule, ultimately resulting in hydroxide and the β–hydroxy aldehyde.
The product obtained when cyclohexanone is heated with a NaOH solution is as shown by the following mechanism:
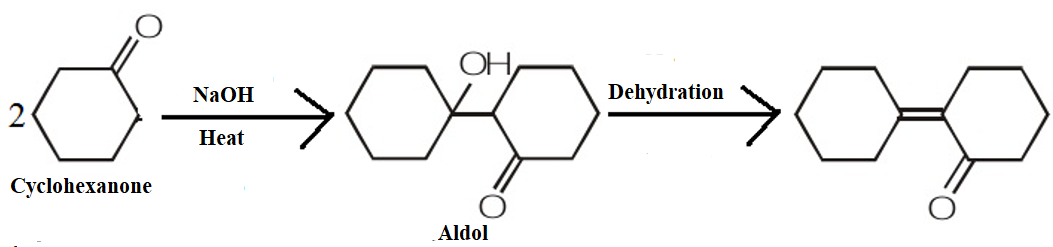
Therefore, we can conclude that the correct answer to this question is option C.
Note:
Make sure you remember the difference between aldol condensation and redox process. Aldox process can be termed as another variation of aldol condensation used in industries for the direct conversion of syngas and propene into 2-ethyl hexanol. The product's formation takes place with hydroformylation of the reactants into butyraldehyde, which further undergoes aldol condensation into 2-ethyl hexenal and the hydrogenation of this intermediate into 2-ethyl hexanol.
