Question
Question: Cyclobutene on oxidation with \[KMn{{O}_{4}}\] gives: A. Succinic anhydride B. Succinic acid C...
Cyclobutene on oxidation with KMnO4 gives:
A. Succinic anhydride
B. Succinic acid
C. Oxalic acid
D. 1,2-dihydroxy cyclobutane.
Solution
The presence of unsaturation in organic molecules can be tested using acidified potassium permanganate (KMnO4) solution. Generally alkenes will give diols with potassium permanganate. But cycloalkenes having high strain means (less than 5 carbons) will give a dicarboxylic derivative as a product.
Complete answer:
The structure of cyclobutene as follows.
The reaction of cyclobutene with potassium permanganate is as follows.
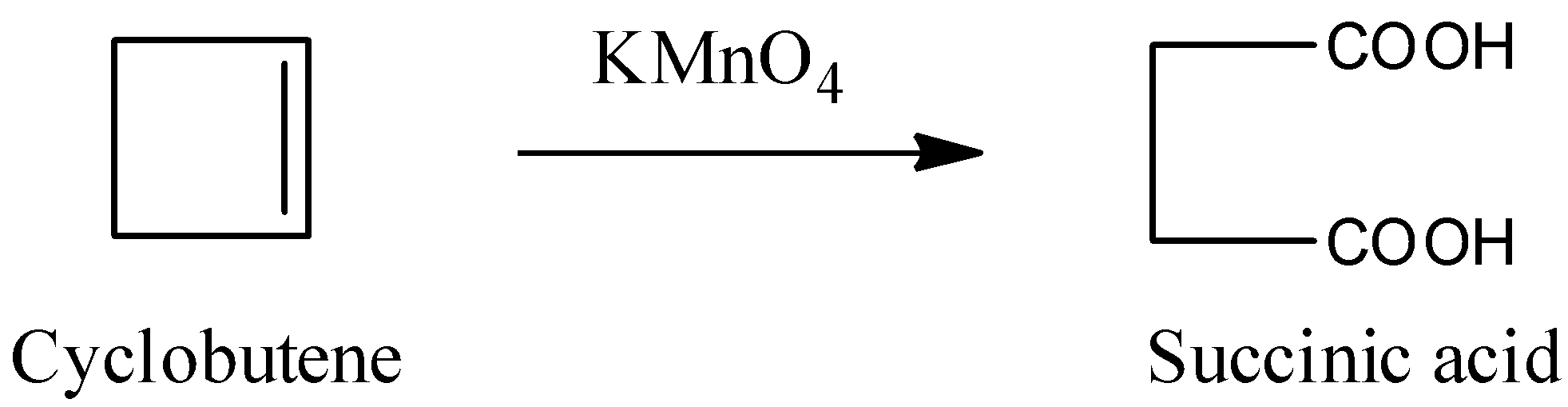
In the above reaction alkene is converted into carboxylic acid.
The product which is formed by the reaction of cyclobutene with KMnO4 is succinic acid.
We can use KMnO4 to prepare small chains of carboxylic acids.
Coming to the given options, option A, succinic anhydride. It is wrong because succinic anhydride is not going to form as a product.
Coming to option C, oxalic acid, it is also wrong oxalic acid contains two carbons in its structure. But the formed product in the above reaction contains four carbons in its structure.
Coming to option D, 1,2-dihydroxy cyclobutane. It is also wrong because in case of cyclobutene hydroxyl groups are not formed when cyclobutene reacts with potassium permanganate.
Coming to option B. Succinic acid, it is correct because succinic acid forms when cyclobutene reacts with potassium permanganate.
So, the correct answer is “Option B”.
Note:
Purple color of KMnO4 (Potassium Permanganate) changes colorless in the presence of unsaturation in the reactants. Also KMnO4 acts as an oxidizing agent in the reaction mentioned in the above solution due to the addition of oxygen atoms to the alkene.
