Question
Question: Cyclobutadiene is said to be: A. Aromatic B. Aliphatic C. Anti-aromatic D. None of the above...
Cyclobutadiene is said to be:
A. Aromatic
B. Aliphatic
C. Anti-aromatic
D. None of the above
Solution
We have the Hückel rule that along with some other rules can help us in deducing the nature of a given compound. Aromatic compounds follow the Hückel rule.
Complete step by step solution
We can classify the organic compounds as aromatic, anti-aromatic and non-aromatic based on the criteria that are discussed here. For an organic compound to be aromatic it has to follow the following criteria:
- Being a cyclic molecule
- Being a planar molecule
- The π electrons are delocalized completely in the ring structure
- Follow the Hückel rule as per which the number of π electrons that are involved in the delocalization can be expressed as (4n+2) where n is an integer.
For example, benzene: Its structure can be shown as below:
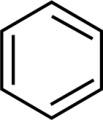
It is a cyclic, planar molecule. There are 6π electrons that can be written as \left\\{ {4\left( 1 \right) + 2} \right\\} = 6 and thus it is an aromatic molecule.
Now, a given compound would be classified as anti-aromatic if it fulfils all the above mentioned criteria except Hückel rule. For example, cyclopentadienyl cation: Its structure can be shown as below:
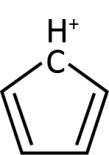
It is also a cyclic and planar molecule. It has 4π electrons that can be written as \left\\{ {4\left( 1 \right)} \right\\} = 4 and thus it is an anti-aromatic molecule.
Now, all the remaining molecules can be simply classified as non-aromatic. Example includes alkanes.
Here, the given molecule is cyclobutadiene. Its structure can be shown as below:

We can see that it is cyclic and planar. It also has 4π electrons that can be written as \left\\{ {4\left( 1 \right)} \right\\} = 4 and thus it is an anti-aromatic molecule.
Hence, the correct option is C.
Note:
We have to carefully look at all the criteria before deducing the nature of the molecule.
