Question
Question: \(CsBr\) crystallizes in a body-centered cubic lattice. The unit cell length is 436.6 pm. Given that...
CsBr crystallizes in a body-centered cubic lattice. The unit cell length is 436.6 pm. Given that the atomic mass of Cs=133 and that of Br=80 amu and Avogadro number being 6.02×1023mol−1 , the density of CsBr is:
A.42.5g/cm3
B.0.425g/cm3
C.8.5g/cm3
D.4.25g/cm3
Solution
Lattice structures are topologically ordered, three-dimensional open-celled structures composed of one or more repeating unit cells [2,3]. These cells are defined by the dimensions and connectivity of their constituent strut elements, which are connected at specific nodes.This question can be solved by using the formula ρ=a3×NAZ×M where Z,M,a,NA,ρ are number of atoms, Mass of consisting atom/molecule, edge length/unit cell length, Avogadro number and density respectively.
Value of Z for BCC is 2.
Complete step by step answer:
Let’s deduce the expression ρ=a3×NAZ×M from the very beginning.
We know density of unit cell =mass of unit cell/volume of unit cell
Mass of a unit cell = number of atoms in unit cell × mass of single atom = Z×m−−−(1) where, Z,m are number of atoms and mass of single atom/molecule in unit cell. We can find the mass of a single atom/molecular by dividing atomic mass M by Avogadro’s Number NA.
So, m=NAM−−−(2)
From equation (1) & (2)
Mass of unit cell =NAZ×M−−−(3)
Now we can write density of unit cell ρ=V×NAZ×M−−−(4) where V is volume of unit cell. As we also know that the volume of a cube is cube of its edge length, consider an edge length a , so volume V=a3.
So, equation (4) can be rewritten as ρ=a3×NAZ×M−−−(5) .
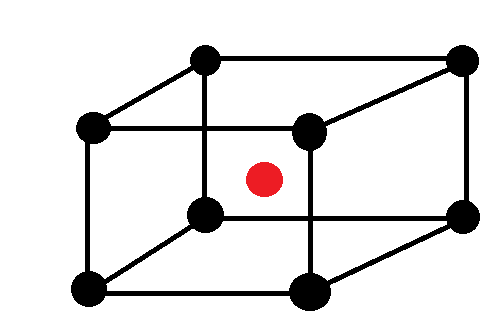
Now, Let’s calculate the number of atoms in the unit cell that is BCC.
Atoms at the corners are 8 and contribution of each corner atom is 1/8 and atom at centre is one and it is not shared with any other cell.
So, Z=8×81+1=2
As per question a=436.6pm=436.6×10−10cm,M=133+80=213g/mol
Putting all values in equation (5) we get,
ρ=(436.6×10−10cm)3×6.02×1023mol−12×213g/mol
⇒ρ=8.5g/cm3
Note:
While deducing the formula ρ=a3×NAZ×M we used m and M , both are different. m is mass of single atom M is atomic mass in amu. So, when we take M atomic mass in grams, according to concept of moles, we get mass of 1 mole of atoms that are equal in Avogadro’s number =6.02×1023mol−1 . So, in order to find the mass of a single atom m=NAM .
