Question
Question: Crystal field stabilization energy for the high spin \({{d}^{4}}\) octahedral complex is: (a)- \(-...
Crystal field stabilization energy for the high spin d4 octahedral complex is:
(a)- −1.8 Δ∘
(b)- −1.6 Δ∘+P
(c)- −1.2 Δ∘
(d)- −0.6 Δ∘
Solution
In octahedral complexes, there is the involvement of d-orbitals, and there are five orbitals of d. When the ligands approach the metal ion, the d-orbitals will split into two parts: t2g and eg. For high spin complexes, the pairing of the electron will not occur.
Complete answer:
The octahedral complexes are formed in the element in which there is the presence of d-orbital or we can say that in octahedral complexes, there is the involvement of d-orbitals, and there are five orbitals of d.
The names of five d-orbitals are dxy,dyz,dzx,dx2−y2, and dz2.
All the energy of the five orbitals are the same but when the ligands approach the metal ion, the d-orbitals of the metal ion will split into two sets: t2g and eg. The t2g part contains the three orbitals and the egpart contains the two orbitals. The value of energy of the t2g orbitals is −0.4 Δ∘ and the value of energy of the eg orbitals is +0.6 Δ∘.
In d4 complexes, there will be 4 electrons in the d-orbital. In the question, the complex is high spin which means that the pairing of electrons will not occur until all the orbitals will get one electron.
For filling of 4 electrons, the three t2g orbitals will get 3 electrons and one electron will enter the e.g. orbital.
The diagram is given below:
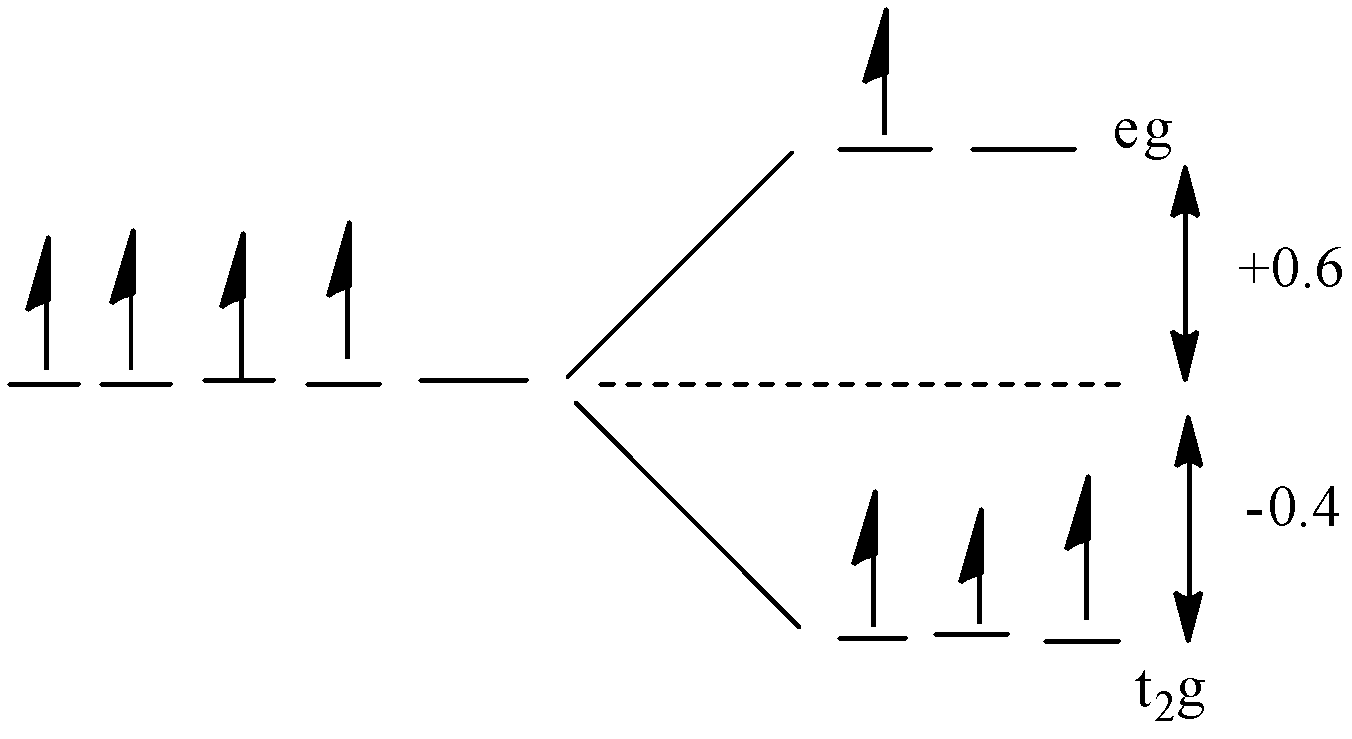
The energy will be:
CFSE=(3 x -0.4)+(1 x 0.6)
CFSE=(−1.2+0.6)
CFSE=−0.6 Δ∘
So, the CFSE (Crystal field stabilization energy) for the high spin d4 octahedral complex will be −0.6 Δ∘.
Therefore, the correct answer is an option (d).
Note:
It must be noted that when the ligand is high spin complex then the pairing of the electron will occur and when the ligand is low spin complex then first the pairing of an electron in t2g set will occur then the electron will move into the eg orbital.
