Question
Question: Cross Cannizzaro reaction is an example of: A) Redox reaction B) Disproportionation reaction C...
Cross Cannizzaro reaction is an example of:
A) Redox reaction
B) Disproportionation reaction
C) Only oxidation reaction
D) Both A) and B)
Solution
The answer here includes about small difference in Cannizzaro and Cross Cannizzaro reaction that in both one molecule gets oxidised and other gets reduced but the aldehydes used are same in Cannizzaro reaction but different in Cross Cannizzaro reaction.
Complete step by step answer:
In the named reaction of organic chemistry part, we have come across the Cannizzaro reaction and are familiar with it.
- Cannizzaro reaction is the one in which two moles of the same aldehyde reacts in the presence of a base to give alcohol and a salt which can be hydrolysed to give an acid.
The reaction with an example of benzaldehyde is as shown below:
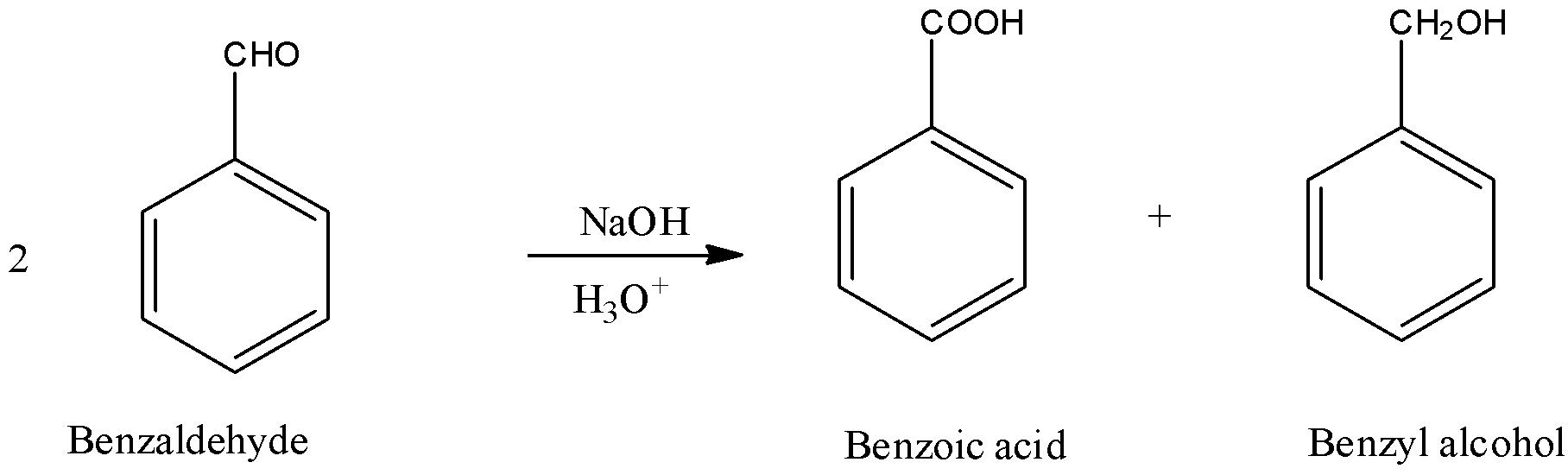
Here, one mole undergoes oxidation to give carboxylic acid and the other mole undergoes reduction to give an alcohol.
Similarly, in cross cannizzaro reaction, the aldehydes taken are different and the reaction with an example of formaldehyde and benzaldehyde in the presence of base is as shown below,
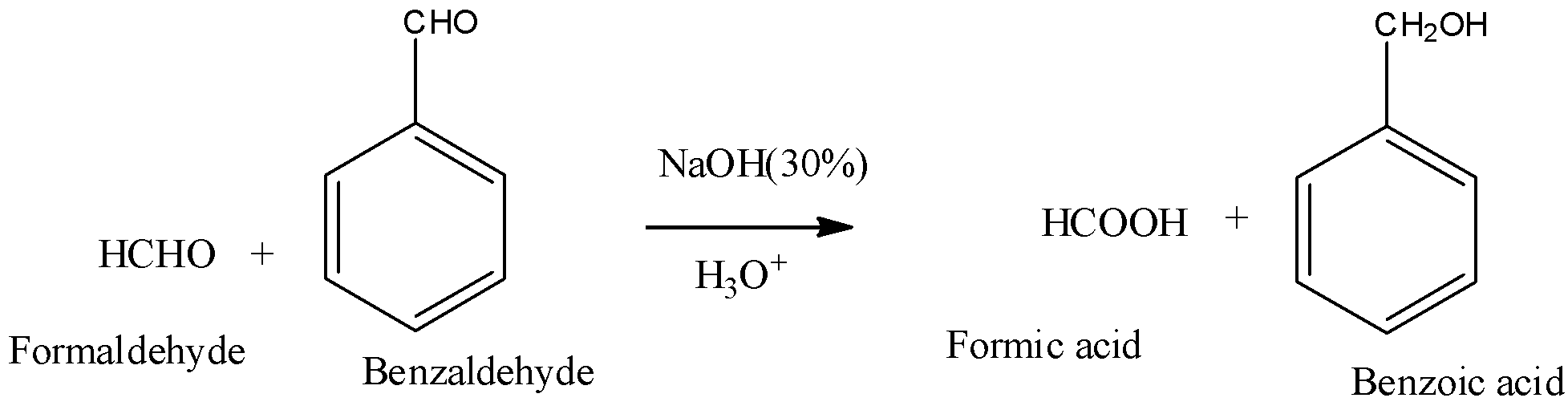
Here the base attacks formaldehyde prior as there are no electron donating groups on it and nucleophilic attack is faster here. Even here Formaldehyde undergoes oxidation to give salt which can be further hydrolysed to give acid and benzaldehyde undergoes reduction to give benzyl alcohol.
Thus, this process of oxidation and reduction in a single step is called redox reaction.
So, the correct answer is “Option A”.
Note: The important point to be concentrated in this is that both the reactions do not contain α−hydrogen as its presence do not give redox products but rather are prone to enolisation which further undergo Aldol condensation.
