Question
Question: \(Cr - C\) bond in the compound \([Cr{(CO)_6}]\) shows \(\pi - \) character due to : A. covalent b...
Cr−C bond in the compound [Cr(CO)6] shows π− character due to :
A. covalent bonding
B. coordinate bonding
C. synergic bonding
D. ionic bonding
Solution
Hint: We have studied in complex compound that metal carbonyls are coordination complexes of transition metals where carbon monoxide (CO) is acts as ligand (ligands are ion, atom or molecule that bonds to central metal, it involves in donation of one or more of its electrons). The important point to mention here is that CO is a neutral and strong field ligand which tends to pairing of electrons. This complex compound is diamagnetic in nature because of strong field ligands. It is d2sp3 hybridized.
Complete answer:
See the following structure of chromium hexacarbonyl in the figure. CO is a π - acceptor ligand ,to form a metal centre it accepts electron cloud density which results in synergistic effect. Cr−C is stronger whereas the C−O bond is weaker. Chromium metal receives electron density via σ-bond and chromium donates back via pi- bond. So in synergic bonding electrons are transferred from ligands to metal. It is bonding between carbonyl groups (ligand) and metal . It is a self strengthening bond. So Cr−C bond in the compound [Cr(CO)6] shows π− character due to synergic bonding.
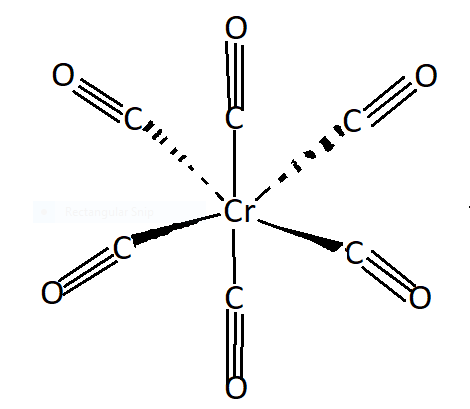
Note: Now we know that Synergic bonding can be named as back-bonding. Due to this bonding carbonyl group donates its pair of electrons to an empty d orbitals, so due to this kind of bonding
Cr−C bond in the compound [Cr(CO)6] shows π− character. We also get some information about the coordinate compound chromium hexacarbonyl. Chromium carbonyl is known as chromium hexacarbonyl. In this complex compound Cr has zero oxidation state .It is a colourless crystal. The coordination geometry of the complex is octahedral.
