Question
Question: Covalency for central atom is maximum in: A.\[B{F_3}\] B.\[S{O_2}C{l_2}\] C.\[POC{l_3}\] D.\...
Covalency for central atom is maximum in:
A.BF3
B.SO2Cl2
C.POCl3
D.BeCl2
Solution
First we have to know the covalency of an atom is the number of bonds an atom forms within a given molecule or compound. To find the covalency, we need to draw the Lewis structure (Chemical structure) of the given molecule and count the number of bonds or the number of shared electron pairs.
Complete answer:
The chemical structure of BF3 is
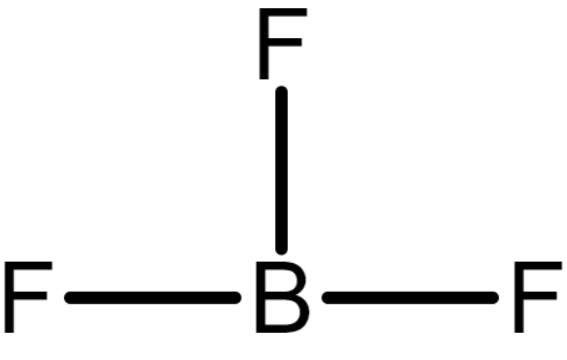
In BF3, the boron atom forms 3 bonds with Fluorine. So, covalency BF3 is 3.
The chemical structure of SO2Cl2 is
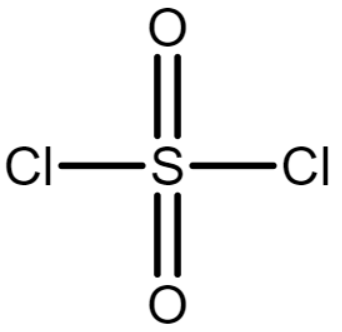
In SO2Cl2, the sulphur atom forms 4 bonds with oxygen and 2 bonds with chlorine. So, covalency SO2Cl2 is 6.
The chemical structure of POCl3 is
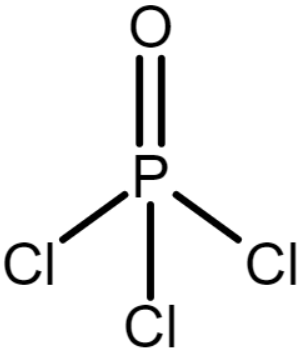
In POCl3, the phosphorus atom forms 2 bonds with oxygen and 3 bonds with chlorine. So, covalency POCl3 is 5.
The chemical structure of BeCl2 is

In BeCl2, the beryllium atom forms 2 bonds with chlorine. So, covalency BeCl2 is 2.
Hence the correct option is (B) SO2Cl2.
Note:
Note that the combining capacity of an atom or a molecule is known as its valency. The valency of an element in a molecule is equal to the number of electrons present in the outermost shell and if it is greater than 4, then the valency of an element is determined by subtracting the total number of electrons present in the outermost shell from eight. If the number of electrons in the outer shell is between one to four, the compound is said to have positive valency.
