Question
Question: Count the total number of unshared electrons in $I_3^- = x$ Count the total number of $p\pi - d\pi$...
Count the total number of unshared electrons in I3−=x
Count the total number of pπ−dπ bonds in PO43−=y
Count the total number of lone pairs in XeF4=z
Hence, find the value of x+y+z
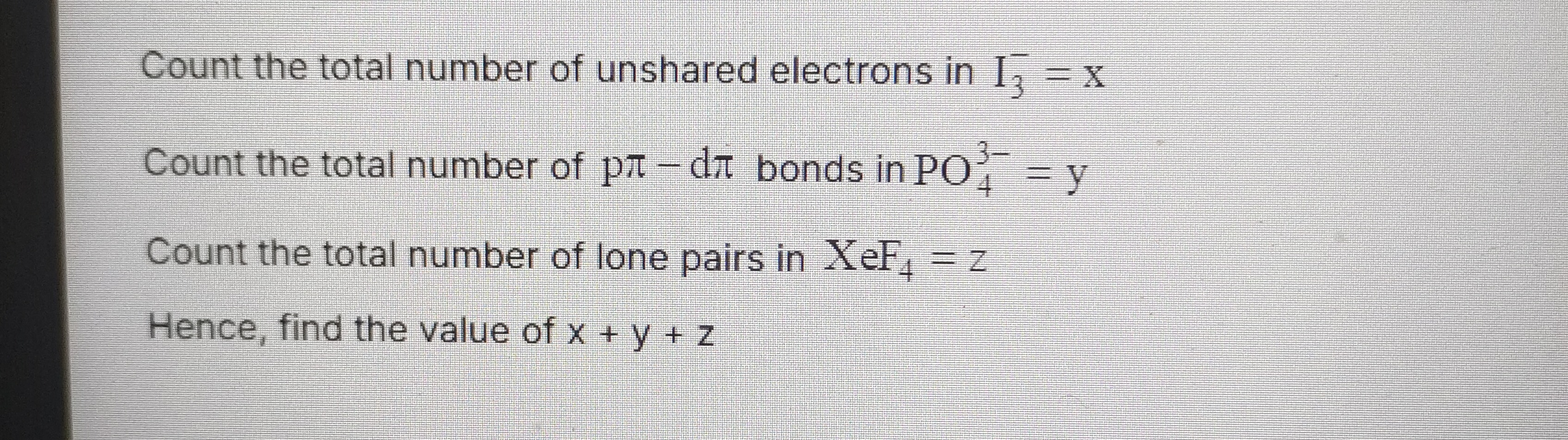
Answer
32
Explanation
Solution
-
For I₃⁻:
- Total valence electrons = 3(7) + 1 = 22.
- Two bonds (each 2 e⁻) use 4 electrons; remaining electrons = 22 − 4 = 18.
- These 18 electrons are present as lone (unshared) electrons on the three iodine atoms.
- Hence, x = 18 electrons.
-
For PO₄³⁻:
- In PO₄³⁻, the best resonance hybrid shows four equivalent P–O bonds.
- Modern understanding shows that effective pπ–dπ bonding is negligible.
- Thus, y = 0 (no effective pπ–dπ bonds).
-
For XeF₄:
- XeF₄ has a square planar structure.
- Xenon: 8 valence electrons; 4 are used in bonding so remaining 4 electrons make 2 lone pairs.
- Each Fluorine: 7 valence electrons; 1 used in bonding so remaining 6 electrons make 3 lone pairs.
- With 4 fluorines, lone pairs on F = 4 × 3 = 12.
- Total lone pairs in the molecule = 2 (on Xe) + 12 (on F) = 14 pairs.
- Hence, z = 14.
Finally, x+y+z=18+0+14=32.
