Question
Question: Correct statements for an element with atomic number 9 are...
Correct statements for an element with atomic number 9 are
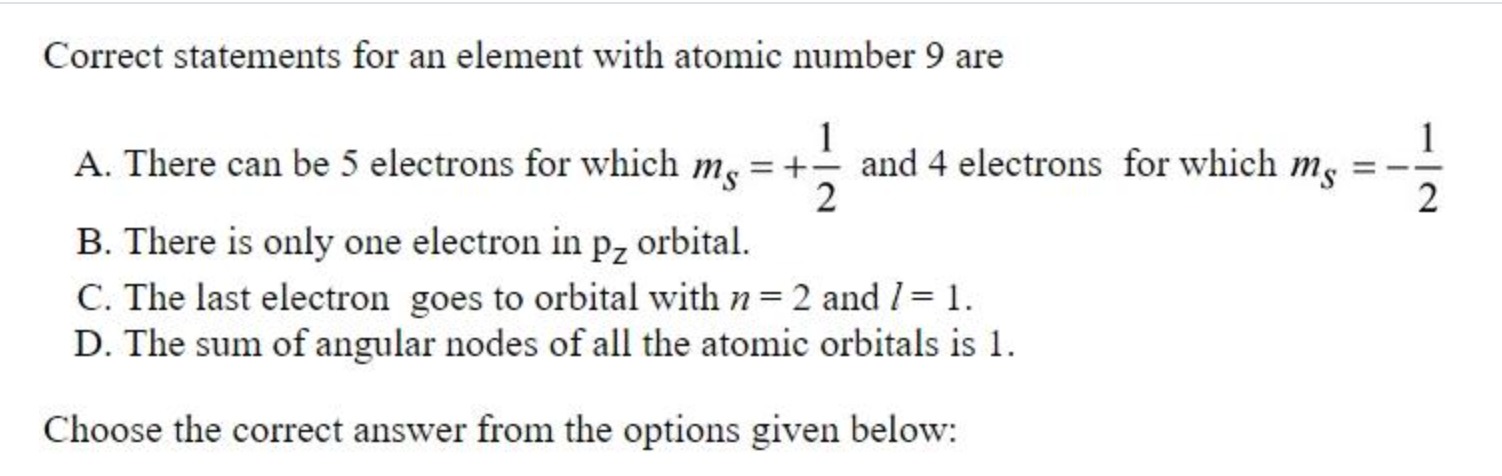
There can be 5 electrons for which ms=+21 and 4 electrons for which ms=−21
There is only one electron in pz orbital.
The last electron goes to orbital with n=2 and l=1.
The sum of angular nodes of all the atomic orbitals is 1.
A, B, and C
Solution
- Electron Configuration:
For atomic number 9 (fluorine), the configuration is:
1s22s22p5- Spin Distribution (Statement A):
- The 1s and 2s orbitals each have 2 electrons (one with ms=+21 and one with ms=−21).
- In the 2p orbitals, the 5 electrons are arranged by Hund’s rule:
- First, one electron in each of the three p orbitals (all with ms=+21).
- Then, the remaining 2 electrons pair up in two of these orbitals (each pairing contributes one electron with ms=−21).
- Count:
- Total ms=+21: 1(1s)+1(2s)+3(firstelectronsin2p)=5
- Total ms=−21: 1(1s)+1(2s)+2(pairedelectronsin2p)=4
So, Statement A is correct.
- Orbital Distribution (Statement B):
In the 2p orbitals, after distributing one electron in each orbital, the remaining two electrons pair up in any two of the three orbitals. By convention (or choice of labelling), it is common to have the pz orbital remain singly occupied. Thus, Statement B is correct.
- Location of Last Electron (Statement C):
The last (9th) electron goes into the 2p orbital. For a 2p orbital, the quantum numbers are n=2 and l=1. Hence, Statement C is correct.
- Angular Nodes (Statement D):
- For s orbitals (l=0), there are no angular nodes.
- For a p orbital (l=1), each has 1 angular node.
While each individual 2p orbital has an angular node, the statement "the sum of angular nodes of all the atomic orbitals is 1" is ambiguous and not meaningful when summing over orbitals with different quantum numbers. Thus, Statement D is incorrect.
