Question
Question: Correct reaction with products are:...
Correct reaction with products are:
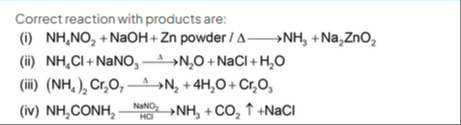
NH4NO2+NaOH+Zn powder/Δ⟶NH3+Na2ZnO2
NH4Cl+NaNO3⟶ΔN2O+NaCl+H2O
(NH4)2Cr2O7⟶ΔN2+4H2O+Cr2O3
NH2CONH2HClNaNO2NH3+CO2↑+NaCl
(iii)
Solution
Let's analyze each reaction:
(i) NH4NO2+NaOH+Zn powder/Δ⟶NH3+Na2ZnO2 This reaction involves the reduction of the nitrite ion (NO2−) by zinc in an alkaline medium. The nitrogen in NO2− (oxidation state +3) is reduced to nitrogen in NH3 (oxidation state -3). The ammonium ion (NH4+) present in NH4NO2 will also react with the strong base NaOH to produce NH3. Zinc is oxidized to zincate (ZnO22−). So, the products NH3 and Na2ZnO2 are expected. However, the given equation is not stoichiometrically correct. The reduction of NO2− to NH3 by Zn in alkaline medium is: NO2−+3Zn+7OH−⟶NH3+3ZnO22−+H2O. The reaction of NH4+ with OH− is NH4++OH−⟶NH3+H2O. Starting with NH4NO2, the overall reaction would involve both processes. The given equation shows a 1:1:1 ratio of reactants producing 1:1 ratio of products, which is incorrect based on balancing redox and acid-base reactions involved. Therefore, reaction (i) is incorrect as written.
(ii) NH4Cl+NaNO3⟶ΔN2O+NaCl+H2O Heating a mixture of NH4Cl and NaNO3 can lead to the formation of NH4NO3 and NaCl through a double displacement reaction: NH4Cl+NaNO3⟶NH4NO3+NaCl. Ammonium nitrate (NH4NO3) decomposes upon heating to produce nitrous oxide (N2O) and water: NH4NO3⟶ΔN2O+2H2O. Combining these, the overall reaction is NH4Cl+NaNO3⟶ΔN2O+NaCl+2H2O. The given equation shows only one molecule of water as a product, which is incorrect. Therefore, reaction (ii) is incorrect.
(iii) (NH4)2Cr2O7⟶ΔN2+4H2O+Cr2O3 This is the thermal decomposition of ammonium dichromate. It is a well-known reaction, often demonstrated as a "volcano" experiment. Let's check if the equation is balanced. Reactants: N = 2, H = 8, Cr = 2, O = 7. Products: N in N2 = 2, H in 4H2O = 4×2=8, Cr in Cr2O3 = 2, O in 4H2O+Cr2O3 = 4×1+3=7. The equation is balanced, and the products are correctly identified. This reaction is correct.
(iv) NH2CONH2HClNaNO2NH3+CO2↑+NaCl This reaction involves urea (NH2CONH2) reacting with sodium nitrite (NaNO2) and hydrochloric acid (HCl). NaNO2 and HCl react to form nitrous acid (HNO2). Urea reacts with nitrous acid to produce nitrogen gas (N2), carbon dioxide (CO2), and water (H2O): NH2CONH2+2HNO2⟶N2↑+CO2↑+3H2O. Since HNO2 is formed from NaNO2+HCl, the overall reaction is: NH2CONH2+2(NaNO2+HCl)⟶N2↑+CO2↑+3H2O+2NaCl. The given equation shows ammonia (NH3) as a product instead of nitrogen gas (N2). Therefore, reaction (iv) is incorrect.
Based on the analysis, only reaction (iii) is correctly represented with the correct products and stoichiometry.
