Question
Question: Correct order of rate of hydrolysis for following compounds is: 
A. I > II > IV > III
B. I > II > II > IV
C. III > I > II > IV
D. III > II > IV > I
Solution
Hydrolysis of alkyl halide is a reaction of alkyl halide with aq. KOH or moist Ag2O. The reaction goes through the SN1 mechanism. A carbocation forms during the reaction. More stable the carbocation more will be the rate of hydrolysis. The stability of the carbocation depends upon the electron donor. So, we will determine the stability of the carbocation for the rate of hydrolysis order.
Complete answer:
In the first step of the hydrolysis, the bromide will remove, so, a carbocation forms. We will check the stability of the carbocation as follows:
As the carbocation is an electron deficient species, the electron donor increases the stability of the carbocation.
The carbocation of each molecule is shown as follows:
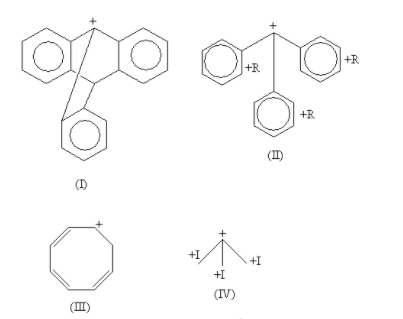
Here, all are tertiary alkyl halides except the compound-III which is secondary.
In the carbocation of compound-I, the carbocation is present at bridge position. According to Bredt's rule a carbocation or double bond is not stable at bridge position, so the carbocation of compound-I is least stable.
In the carbocation of compound-II, the carbocation is stabilised by the electron donating +R effect of three phenyl rings. So, the carbocation of compound-II is stabilised by the resonance effect.
In the carbocation of compound-III, the 6\pi electrons are present. So, it is an aromatic compound according to the Huckel rule but it also has an sp3hybridised carbon so, it is a homo-aromatic compound. So, the carbocation of compound-III is stabilised by the aromatic effect.
In the carbocation of compound-IV, the carbocation is stabilised by the electron donating +I (inductive) effect of three methyl groups. So, the carbocation of compound-IV is stabilised by the inductive effect.
The stabilizing order of different effects to carbocation is as follows:
Carbocation stability ∝ Aromatic > + Resonance effect > + Inductive effect.
So, the stability of the carbocation of the given molecules is as follows:
III > II > IV > I
The rate of hydrolysis is directly proportional to the stability of the carbocation so, the order of rate of hydrolysis is as follows:
III > II > IV > I
Therefore, from the above explanation the correct option is (D) III > II > IV > I.
Note: 1o Alkyl: When a halogen atom is attached to the carbon which is attached only with one carbon. 2oAlkyl: When a halogen atom is attached to carbon which is attached with two other carbons. 3o Alkyl: When halogen atom attached to carbon which is attached with three other carbons.The stability order for carbocation is 3o > 2o > 1o. The compound-II, having carbocation attached with three phenyl rings is known as miscellaneous carbocation. The miscellaneous carbocation is less stable than aromatic carbocation. The stability of carbocation is directly proportional to the electron withdrawing groups.
