Question
Question: Correct order of percentage of enol content is ? 
A.b>d>c>a
B.d>b>c>a
C.b>d>a>c
D.a>b>d>c
Solution
Firstly we have to simplify and mention what exactly is enol. Then in the next step we have to define the existence and the reason for the keto-enol tautomerism. Then we have to mention the reasons we use to determine the stability of these states in order to obtain the right amount of enol content. Then to conclude we have to give the answer and the reason we have used for the stability of the given states.
Complete step by step answer:
Enol refers to an organic compound that contains a hydroxyl group bonded to a carbon atom having a double bond and that is usually characterized by the grouping C=C(OH) . They generally exist in the constant tautomeric state with the ketones. ( A condition where the interconversion takes place to achieve most stable state of the compound referred to the chemical structure and bonding )
This is also called keto-enol tautomerism. This is determined by various factors which participates in determining the stability of the compound like :
i.Resonance
ii.Aromaticity
iii.Hyperconjugation
iv.Polarization and many others.
The amount of keto or enol content depends on the stability of the state.
In the given question, the order of the enol content is drawn by using this logic:
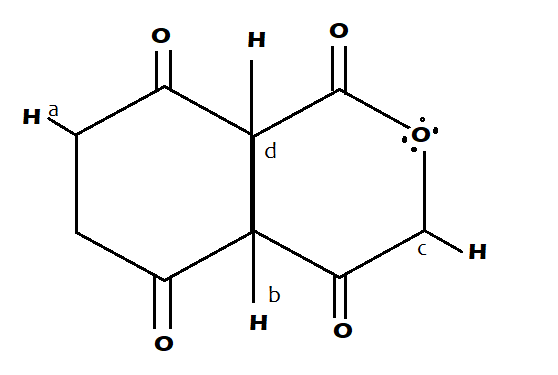
carbon with electron withdrawing groups > carbonyl carbons > no. of alpha hydrogens.
Therefore the correct answer would be B, d>b>c>a.
Note:
When the keto and the enol form exist, although both are stable, but they constantly interconvert in each other to gain much stability and the correct amount of saturation. Even if one form is more stable doesn’t mean that every molecule converts in it. Constant interconversion stables the content.
