Question
Question: Correct order of electrophilic substitution reaction? 
A. a>b>c>d
B. d>b>a>c
C. b>a>c>d
D. b>a>d>c
Solution
Hint: When we consider electrophilic substitution reaction there is an electrophile which attacks the substrate in search of electron density. If the substrate has larger electron density it will be readily attacked by the electrophile. So to solve this question we just have to find the order of electron density and the order of electrophilic substitution reaction will follow the same pattern; that is more electron rich substrate will show greater rate for electrophilic substitution reaction.
Complete step by step solution:
To solve this question we have to find the order of electron rich compounds because more electrons will react more readily in electrophilic substitution reactions.
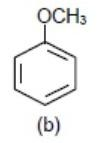
We know −OCH3 (Methoxy group) donates electrons density to benzene ring via+M (mesomeric effect) so the benzene ring with −OCH3 group will be most electron rich so will show fastest reaction.
Also, −CH3(methyl group) donates electrons to benzene ring hy + HC (hyper conjugation) effect and it will make benzene ring electron rich but not more than −OCH3 group.
Now – Cl (Chlorine) pulls away electron density from the benzene ring via – I effect (inductive effect). So, it will make the benzene ring electron deficient for electrophilic substitution reaction.
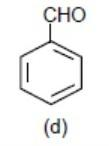
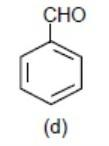
Lastly, we have benzaldehyde, here – CHO (aldehyde group) will withdraw the element density from the benzene ring via – M (mesomeric effect) and make the benzene ring electron deficient.
So this will be least reactive among all.
Hence we get the element density order as
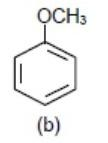 >
>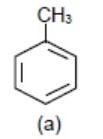 >
> >
>
The order of reactivity for electrophilic substitution reaction will be as follows.
 >
>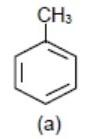 >
>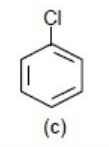 >
>
Hence, the correct option C which is b>a>c>d
Note: A substitution reaction is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. It is majorly of two types electrophilic substitution reaction and nucleophilic substitution reaction. In an electrophilic substitution reaction an electrophile attacks on the Nucleophilic substrate and in nucleophilic substitution reaction nucleophile attacks on an electrophilic substrate.
