Question
Question: Correct order for stability for carbocation is:- (A)  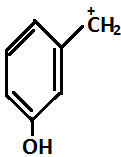
(B) 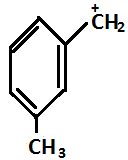
(C) 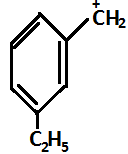
(D) 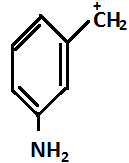
Solution
To solve this question, we should know about the inductive effect, its types, how it affects the stability of an organic compound, the role of electron withdrawing groups and electron releasing groups present in the chain of the carbon atoms.
Complete step by step answer:
Let us understand from the basics.
Firstly, we will know what is the inductive effect and its types.
When the electrical charge is transmitted throughout the chain of atoms, the effect produced is called as an inductive effect. It is a permanent effect. There is an interesting fact to know about inductive effects. It is distance dependent. The relation is as follows:
Inductive effect α distance1
It is of two types-
+I effect: it is also called e− donating effect or e− repelling effect. It enhances the stability. The order for some groups is as follows:
−−CH2>−−NH>−O− >Carboxylic group > 3∘ -alkyl group > 2∘ -alkyl group> 1∘ -alkyl group > -H
–I effect: it is also called e− withdrawing effect or e− attracting effect. The order for some groups is as follows:
−+NR3>−+NH3>−NO2>−SO2R>−C≡N>−CHO>−COOH>−F>−Cl>−Br>−I>−OR>−OH.......>−CH=CH2>−H
So in the given question, (P) and(S) show –I effect and (R) and (Q) show +I effect.
On the basis of the above mentioned order, the decreasing order for stability is R>Q>P>S.
Note:
-I effect decreases e− density on a molecule, making it e− deficient and highly acidic. Also, we should remember the series and difference between both the effects because otherwise the solution may have manual error.
