Question
Question: Correct energy profile for amine inversion and hybridization of nitrogen in transition state is: ...
Correct energy profile for amine inversion and hybridization of nitrogen in transition state is:
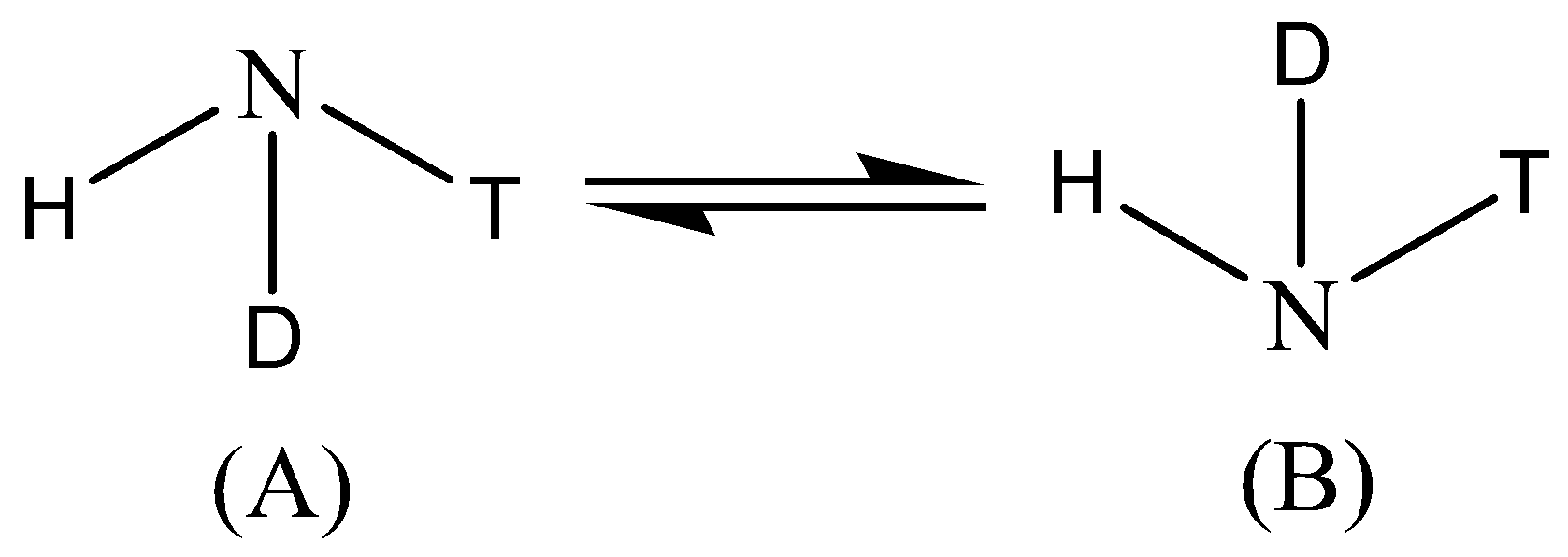
A.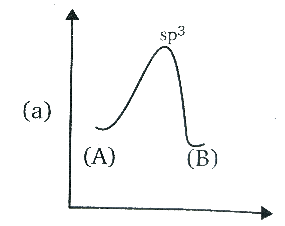
B.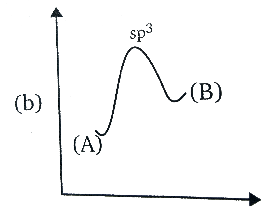
C.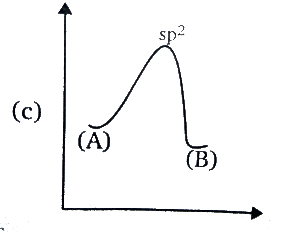
D.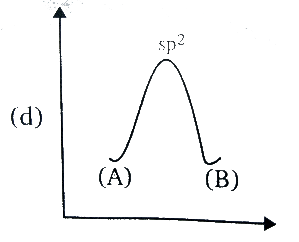
Solution
We know that when an amine group undergoes the process of inversion, the groups that are attached to the nitrogen atoms turn upside down i.e. their special arrangement inverts. The C3 axis of the amine is presented as horizontal, and the dots that are in a pair, they represent the lone pair of the nitrogen atom collinear with that axis.
Complete answer:
Amine has the Sp3 hybridisation, since one ‘s’ orbital and three ‘p’ orbitals are involved in the bonding. During the inversion of the amine, the C3 axis of the amine is presented as horizontal, and the dots that are in a pair, they represent the lone pair of the nitrogen atom collinear with that axis.
In the transition state of amine, it has Sp2 hybridisation, as the lone pair enters the nucleus which leaves behind a vacant orbital of amine. And after sometime, during the process of inversion, the lone pair pops out of the either side, since the inverted amine becomes the mirror image of each other or we can say it becomes an enantiomer, the energy remains the same as the process do not release or absorb the energy.
In the option (D), the graph represents the same energy levels of the (A) and (B) and has Sp2 hybridisation, hence it is correct.
Therefore, the correct answer is option (D).
Note:
It should be noted that the amine’s hybridisation change from Sp3 to Sp2 hybridisation during the transition state of amine or the process of inversion of amine as the lone pair of the nitrogen leaves the atom and creates a vacant space.
