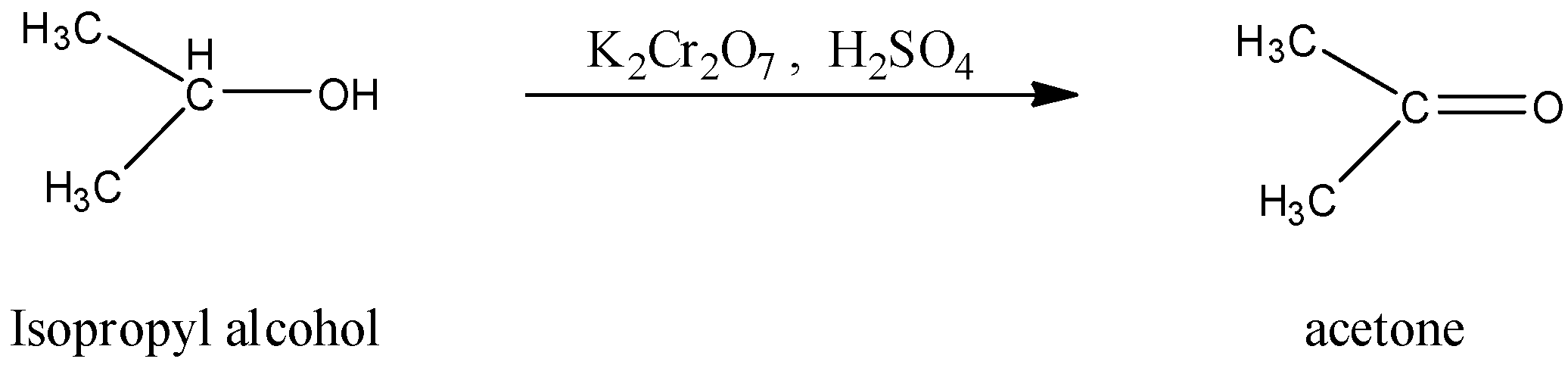Question
Question: Convert (i)- 3-Phenyl-1-Propanol to 3-Phenyl-1-Propanal (ii)- Isopropyl alcohol to acetone....
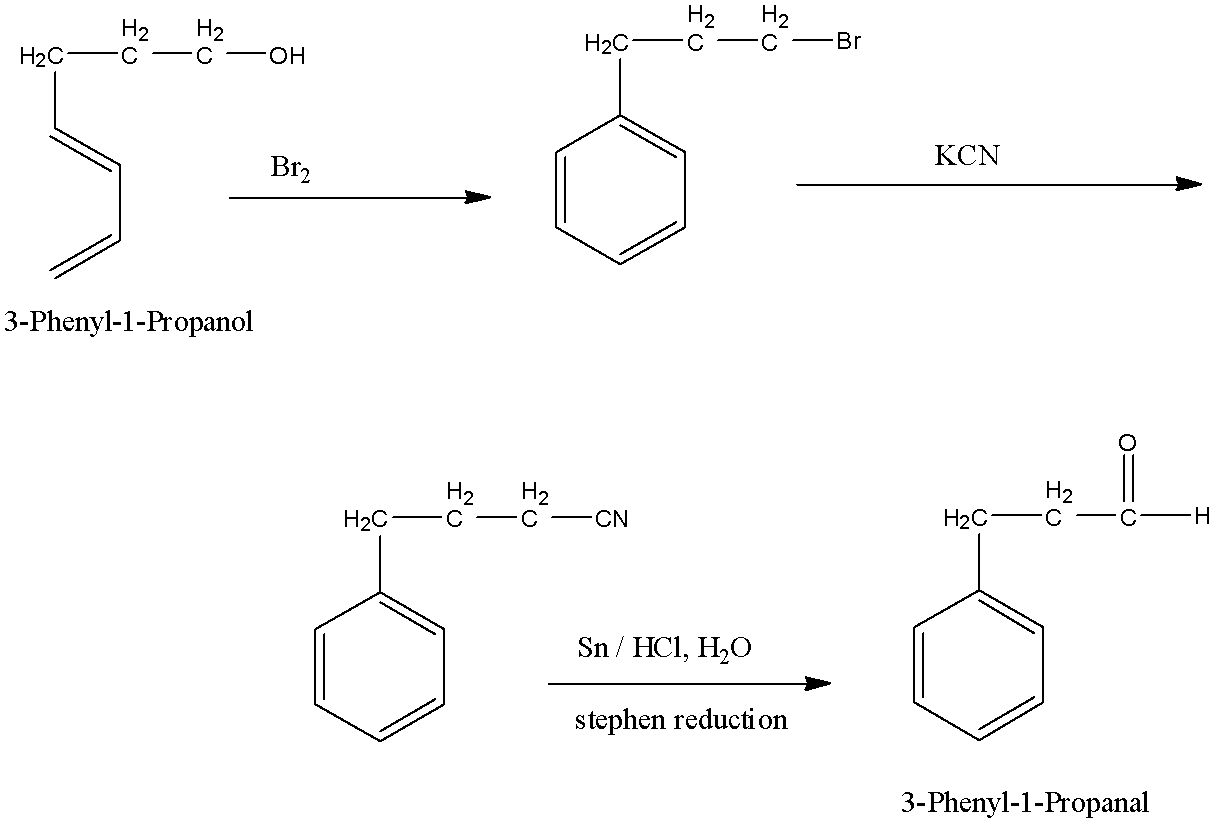
Convert (i)- 3-Phenyl-1-Propanol to 3-Phenyl-1-Propanal
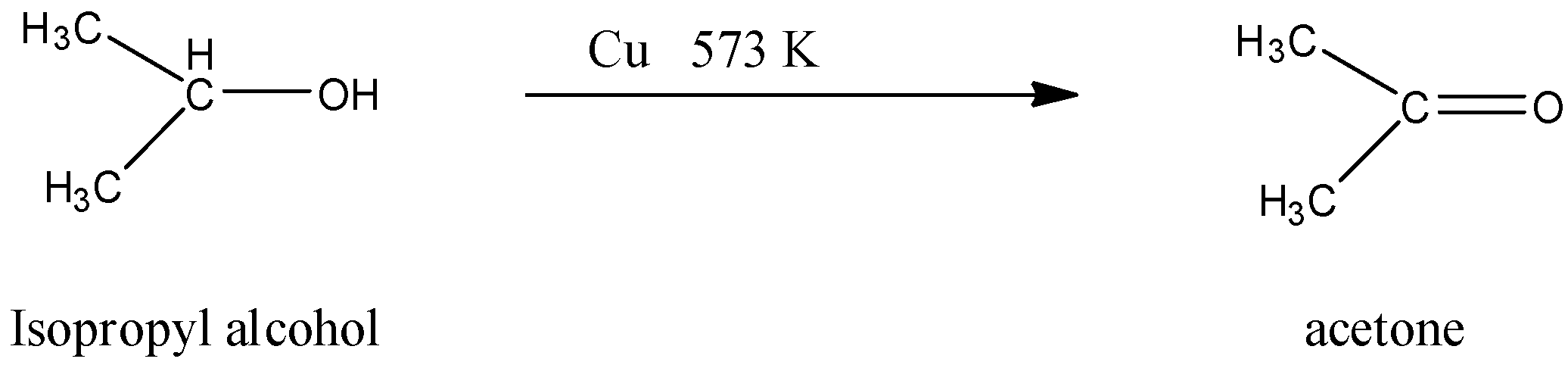
(ii)- Isopropyl alcohol to acetone.
Solution
Alcohol is a functional group which can be converted by aldehyde by oxidation because aldehyde has one hydrogen atom less than the alcohol group. In 3-Phenyl-1-Propanol the alcohol group is converted into halogen, then it is converted into a cyanide group and finally into aldehyde by Stephen reduction.
Complete step by step answer:
(i)- 3-Phenyl-1-Propanol to 3-Phenyl-1-Propanal
3-Phenyl-1-Propanol is an organic compound in which the phenyl group is attached to the 3rd carbon atom of the propanol group and an alcohol group is present at 1st carbon atom. 3-Phenyl-1-Propanal is an organic compound in which the phenyl group is attached to the 3rd carbon atom of the propanol group and an aldehyde group is present at 1st carbon atom.
3-Phenyl-1-Propanol can be converted into 3-Phenyl-1-Propanal by some steps as First, the alcohol group is converted into halogen, now this halogen is converted into cyanide group by the action of potassium cyanide. Now, this cyanide group on reduction forms aldehyde by the action of stannous chloride and hydrogen chloride (it is also known as Stephen reduction). The reactions are given below:
(ii)- Isopropyl alcohol to acetone.
Isopropyl alcohol is an organic compound in which secondary alcohol is present and acetone is an organic compound, which is a 3 carbon chain and the second carbon atom has the ketone group. The Isopropyl alcohol can be directly converted into acetone by oxidation with copper at 573 K. The reaction is given below:
Note: Not only by copper, but Isopropyl alcohol can also be converted into acetone by oxidizing it with potassium dichromate in the presence of sulfuric acid. The reaction is given below: