Question
Question: Convert Ethanol to But-1-yne....
Convert Ethanol to But-1-yne.
Solution
First, convert the alcohol into a haloalkane. Now take acetylene and convert it into sodium acetylide. Now add the haloalkane and sodium acetylide which will form an alkyne containing a higher number of a carbon atom.
Complete step by step answer:
Ethanol is a compound of alcohol groups having a −OHfunctional group. Its formula is CH3−CH2−OH. It is a 2 carbon compound.
But-1-yne is a compound of the alkyne group having a triple bond. Its formula is HC≡C−CH2−CH3. It is a 4 carbon compound.
When alcohols are reacted with hydrobromic acid in the presence of little sulfuric acid it forms bromoalkane.
When acetylene or ethyne is reacted with sodamide in liquid ammonia it forms sodium acetylide. Now, this sodium acetylide can be converted into the required alkyne by adding the haloalkane of the required number of a carbon atom.
To convert ethanol to but-1-yne:
We have to convert a 2 carbon compound to a 4 carbon compound.
First, convert the ethanol to bromoethane with hydrobromic acid and a little amount of H2SO4 Next, take acetylene and react with NaNH2 to form sodium acetylide. Now, add the bromoethane and acetylide to form but-1-yne. This reaction is useful for making higher alkynes have triple bonds at the first carbon atom.
The reaction and mechanism are given below:
CH3−CH2−OH→HC≡C−CH2−CH3
The mechanism of the reaction is:
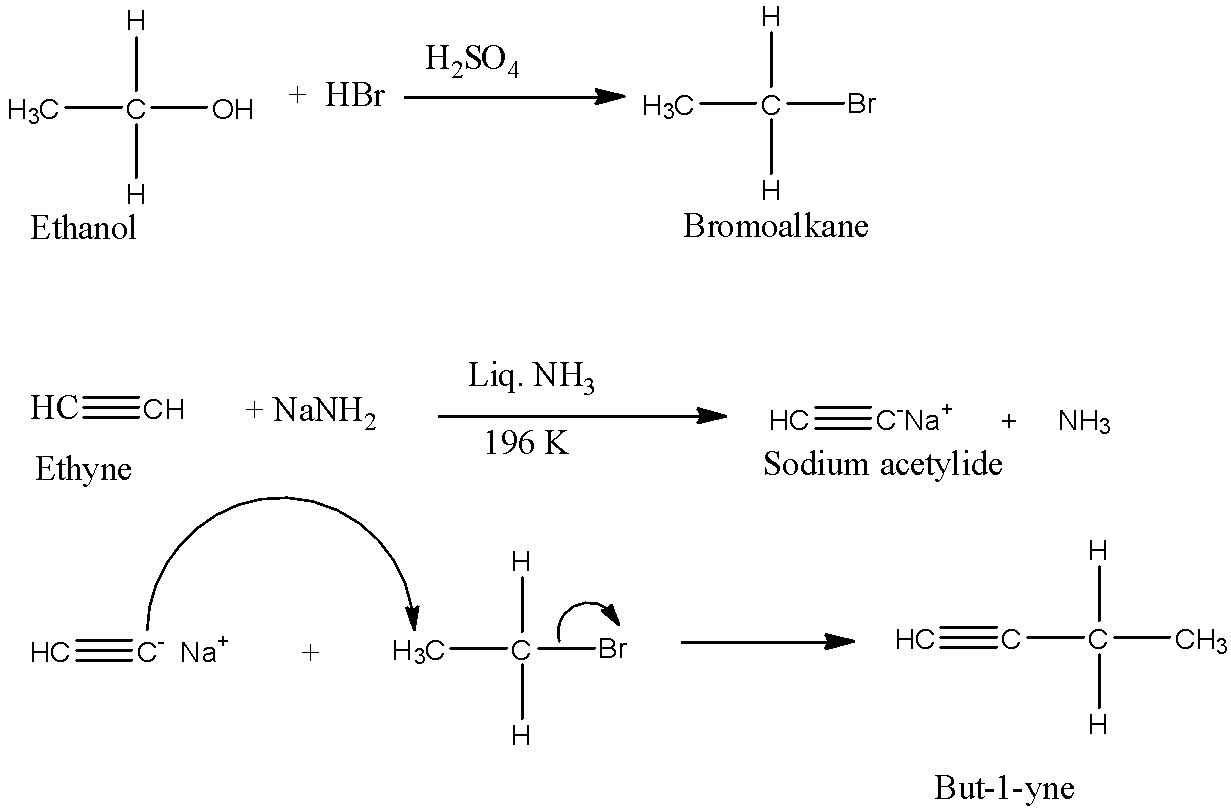
Note: When 2 moles of NaNH3 are reacted with the ethyne or acetylene, both the hydrogen of the ethyne are replaced with the sodium atom. Now, this sodium acetylide can be used to make alkynes in which the triple bond is not at the first carbon atom.
