Question
Question: Convert ethanal to isopropyl alcohol....
Convert ethanal to isopropyl alcohol.
Solution
Before moving to the steps involved in the conversion of ethanal to isopropyl alcohol, let’s know about both of the compounds. Ethanal is an aldehyde having two carbon atoms since it is ended with the term “al” and starting with the term “eth”. Isopropyl alcohol is a secondary alcohol with a total of three carbon atoms.
Complete answer:
The compounds which have a −CHO group are called aldehydes. Carbon is double bonded to oxygen, singly bonded to R group and hydrogen. R group may be hydrogen, alkyl group or any aryl group. Alcohol is a compound in which a hydroxyl group is attached to a carbon atom. As we have said that isopropyl alcohol is a secondary alcohol. Secondary alcohol is the alcohol in which a hydroxyl group is attached to a carbon atom which is further attached to other two carbon atoms.
The steps involved in the reaction are given below:
Ethanal is a compound which has two carbon atoms and the product isopropyl alcohol has three carbon atoms. This is made by reacting ethanal with Grignard reagent. The chemical formula of Grignard reagent is RMgBr, where R is methyl group. From this Grignard reagent, one carbon is added to ethanal molecules.
The chemical reaction is given below:
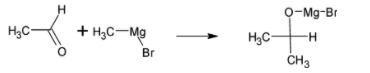
The intermediate undergoes hydrolysis to give isopropyl alcohol. The reaction is given below:
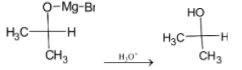
Note:
We can also convert the isopropyl alcohol to a ketone group. Any oxidizing agents like potassium dichromate, sodium dichromate, potassium permanganate or pyridinium chlorochromate can be used to convert to ketone. Propanone is obtained using this reaction.
