Question
Question: Convert Cyanide to Aldehyde and Ketone....
Convert Cyanide to Aldehyde and Ketone.
Solution
Cyanides are compounds which contain R−CN groups in its structure where R is an alkyl group. The general representation of aldehyde and ketone are
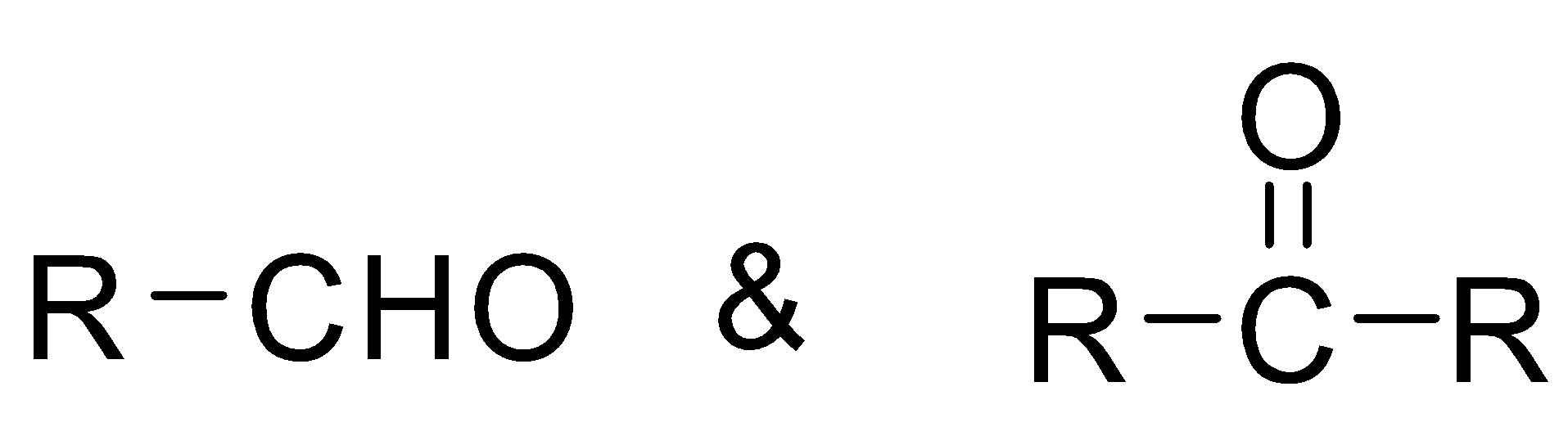
Complete step by step answer:
As we know cyanide is a chemical compound containing C≡N group. This C≡N group is known as the cyano group. The general representation of cyanide is as R−CN where R is an alkyl group. The organic cyanides are known as nitriles. Cyanides are produced by algae, fungi and contain bacteria. Cyanides are found in certain fruit stones and seeds. Cyanides are used in metallurgy of gold and silver. Cyanides help dissolve the metals and there are and this process of concentration of area is known as leaching. Cyanide compound is also used in many mediums.
The conversion of cyanide into aldehyde takes place by reacting a given cyanide with a DIBA L-H [Diisobutylaluminium hydride ] and water which leads to the formation of an aldehyde .
R−CN(1)DIBAL−H(2)H2OR−CHO
Now we have to convert cyanide into ketone is a function group with general representative 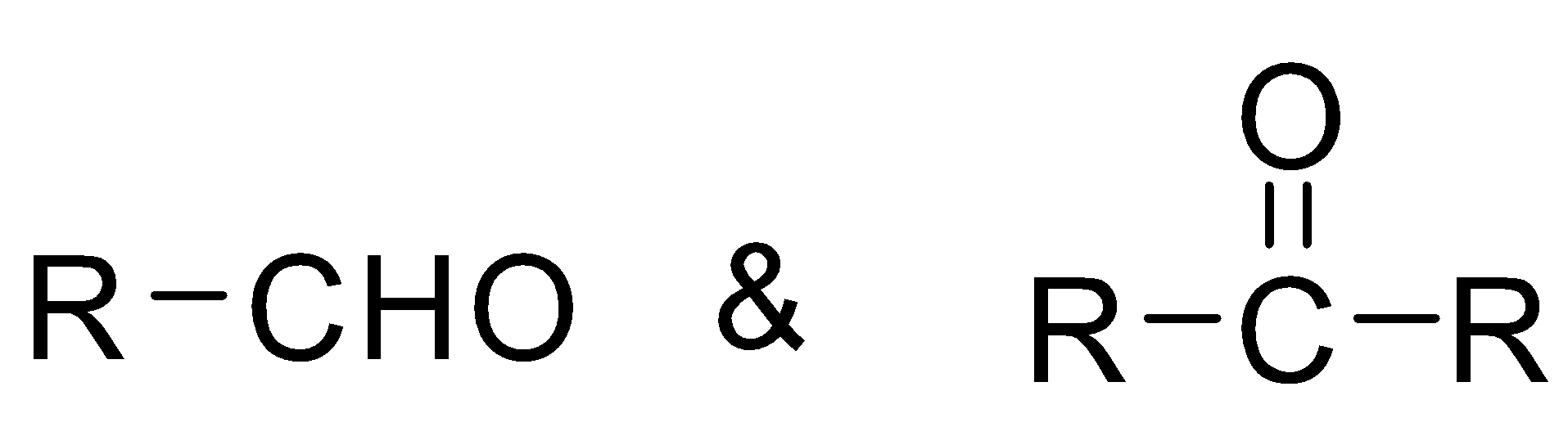
Here R and R’ can be alkyl groups.
The conversion of cyanide into ketone takes by first reacting cyanide with Grignard reagent followed by treating the formed product with water. The reaction takes place as follow:
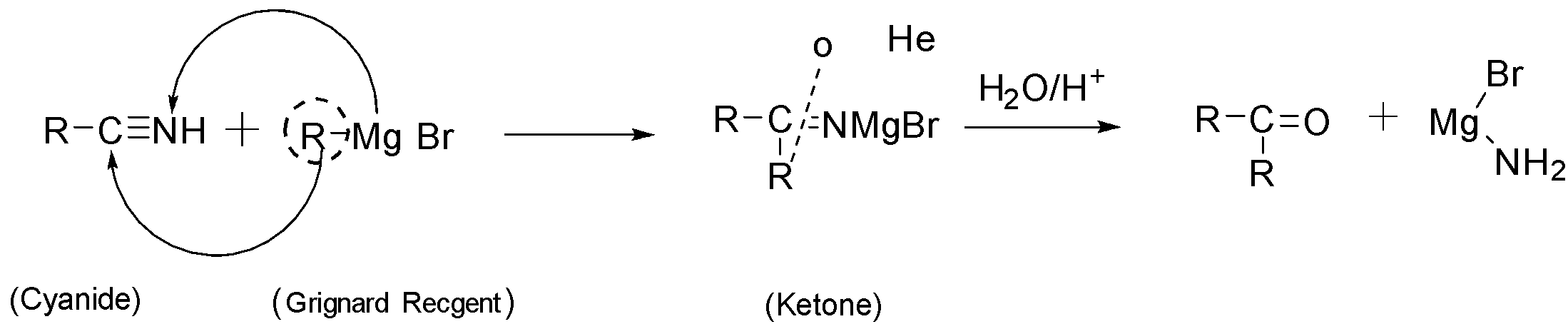
Note: Since, ketone contains more carbon than the cyanides. So we have to extend the chain of starting cyanide which can be done by treating cyanide with suitable Grignard reagent. So Grignard reagent in the conversion of ketone from cyanide is very important.
