Question
Question: Convert cyanide to aldehyde and ketone....
Convert cyanide to aldehyde and ketone.
Solution
In order to convert cyanide to aldehyde and ketone, we need to remove nitrogen atom and add oxygen atom in order to get the desired product. Both the conversions involve imines as intermediate products.
Complete step by step answer:
Here, we are given to convert the cyanide to aldehyde and ketone.
- We know that cyanide is the functional group which involves C-N triple bond and it is expressed by –CN. Now, the aldehyde and ketone functional groups do not have any nitrogen atoms. So, we will need to remove that nitrogen atom and add oxygen atom in order to achieve this conversion.
Cyanide to Aldehyde:
- We can use DIBAL-H reagent to give this conversion. DIBAL-H stands for diisobutyl aluminum hydride. It has a hydride donor and a strong reducing agent. It is able to reduce −C≡N functionality. It will react with the cyanide group as shown below.
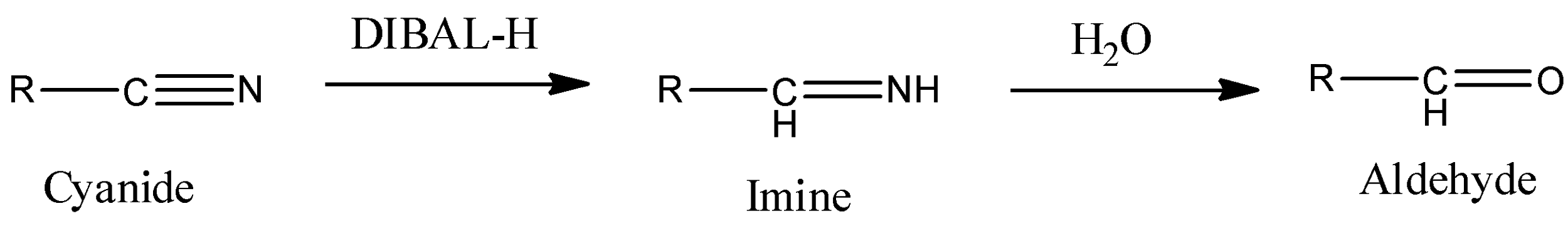
Here, we can see that DIBAL-H reduces −C≡N and gives imine as a product. Then imine is hydrolyzed to get aldehyde as a final product.
Cyanide to ketone:
- In order to convert cyanide to ketone, we need to react Grignard reagent with cyanide. The reaction is given below.
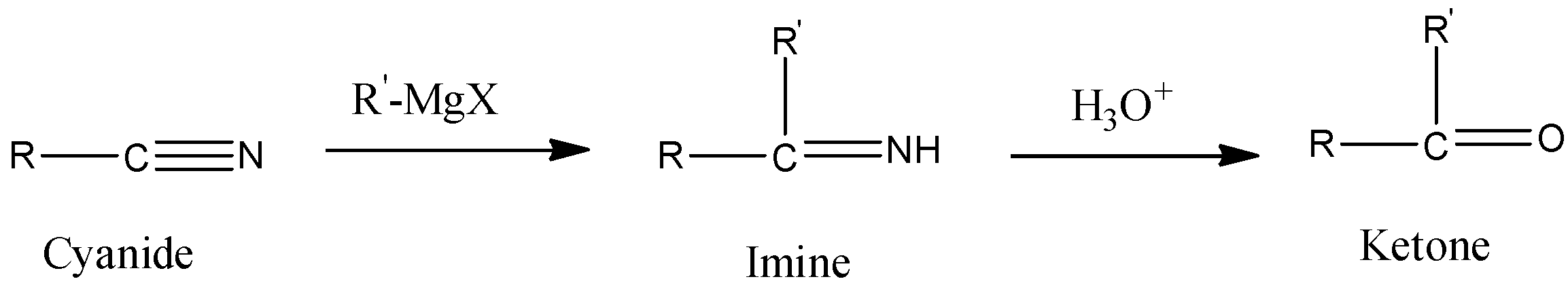
Here, we can see that nucleophilic attack of the alkyl group of Grignard reagent on the electrophilic carbon atom of cyanide gives imine. We know that imine gets hydrolyzed to give aldehydes and ketones. Here, it will give ketones.
Note: Remember that with DIBAL-H, we will always obtain aldehyde as a product as there is only one alkyl group attached with the carbonyl carbon. If we allow that aldehyde to react with Grignard reagent, then we will obtain an alcohol and oxidation of that alcohol with PCC (Pyridinium Chlorochromate) will give us ketone.
