Question
Question: Convert Benzaldehyde to \[2 - \] phenyl nitroethene....
Convert Benzaldehyde to 2− phenyl nitroethene.
Solution
Conversions in organic chemistry are called organic conversions in which one organic compound is converted into another organic compound by using multiple organic compounds or specific reagents. To convert Benzaldehyde to 2− phenyl nitroethane we need to increase the carbon chain.
Complete step by step answer:
First, we will understand some basics of conversions of organic compounds. Organic conversions are similar to chemical reactions. As in the chemical reactions, we have reactants and products here also we follow a similar concept. Now we are coming back to our question. According to the question, we need to convert benzaldehyde to 2− phenyl nitroethene. So here benzaldehyde is the reactant and with the help of a suitable reagent, we will be bringing the conversion between Benzaldehyde to 2− phenyl nitroethene. The conversion of Benzaldehyde to 2− phenyl nitroethane can be explained as below.
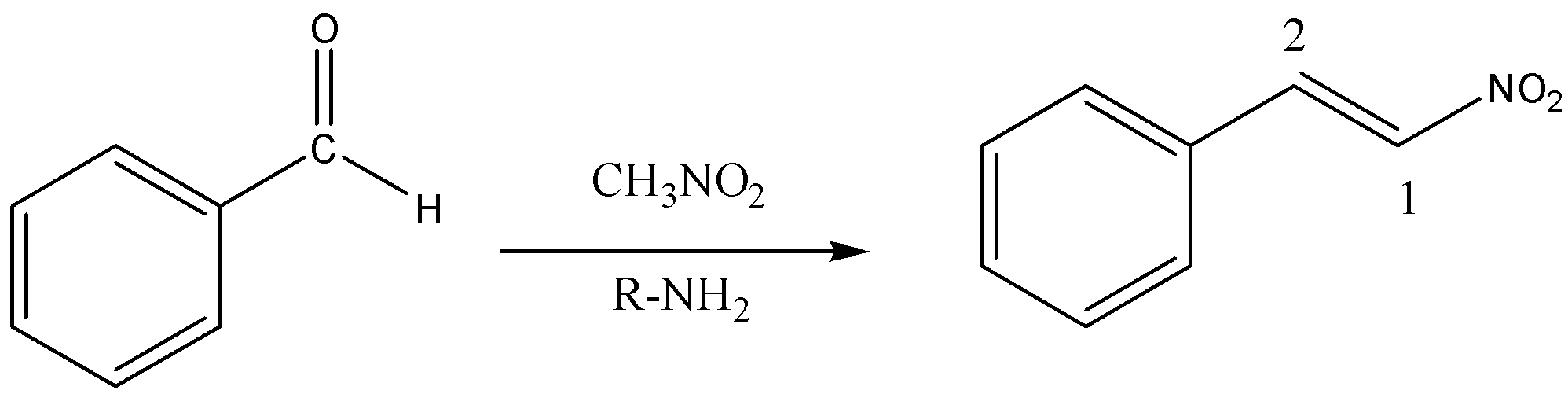
Now we will discuss the above conversion step by step. We need to convert benzaldehyde and the chemical formula of benzaldehyde is C6H5CHO. If we react benzaldehyde with the reagent nitromethane CH3NO2 and primary amine R−NH2 the required product is formed in one step or single step. During the reaction, a water molecule −H2O is removed and the carbon-carbon double bond is formed with the attachment of the nitro group. Hence, the required product is 2− phenyl nitroethene.
Additional information:
The IUPAC name CH3NO2 is nitromethane. It is a useful solvent for polymers such as polystyrene and particularly it is useful for dissolving cyanoacrylate adhesives. One of the most important uses of nitromethane is that it acts as an artificial nail remover.
Note:
The reaction of aldehyde or ketones with the nitroalkanes like nitromethane is known as the Henry reaction. Henry reaction is the base-catalyzed carbon-carbon bond-forming reaction between aldehydes and nitroalkanes. It is similar to the Aldol addition.
