Question
Question: Convert 1 - chloropropane into following: A.\[Propan - 1 - ol\] B.\[1 - iodopropane\] C.Butane...
Convert 1 - chloropropane into following:
A.Propan−1−ol
B.1−iodopropane
C.Butane nitrile
D.Propyl ethanoate
Solution
Conversion: When a chemical reaction takes place, the process of converting takes place and some potion disappears and is converted to other products. The conversion of 1−chloropropane into the following compounds are simple organic reactions. 1−chloropropane (Also known as n-propyl chloride or1−propyl chloride) is an organic compound. The chemical formula for 1−chloropropane is C3H7Cl.
Complete step by step solution:
When the 1−chloropropane is reacted with alcoholic potassium hydroxide,KOH, to give the product Propan−1−ol, the chemical formula for Propan−1−ol is C3H7OH and potassium chloride,KCl, is the byproduct for this reaction.
We can write the chemical equation for this chemical reaction as,
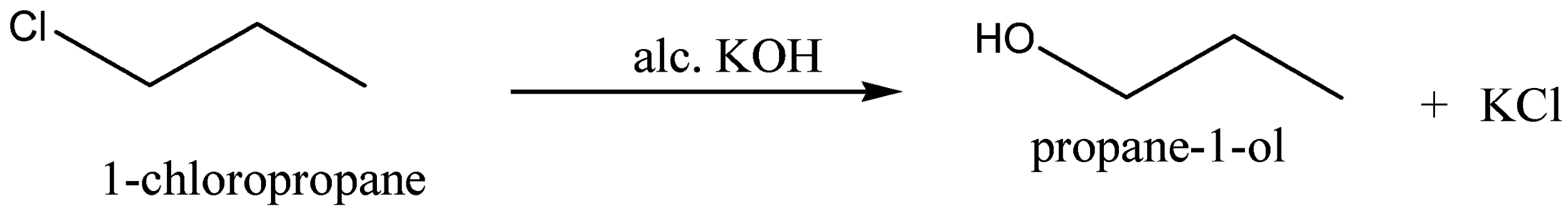
Now we can discuss the conversion of 1−chloropropane into 1−iodopropane.
Now we discuss the conversion of 1−chloropropane into Butane nitrile.
1−chloropropane is reacted with sodium iodide,NaI, to give the corresponding product 1−iodopropane, (chemical formula: C3H7I) and also give sodium chloride,NaCl, as byproduct.
We can write the chemical equation for this chemical reaction as,
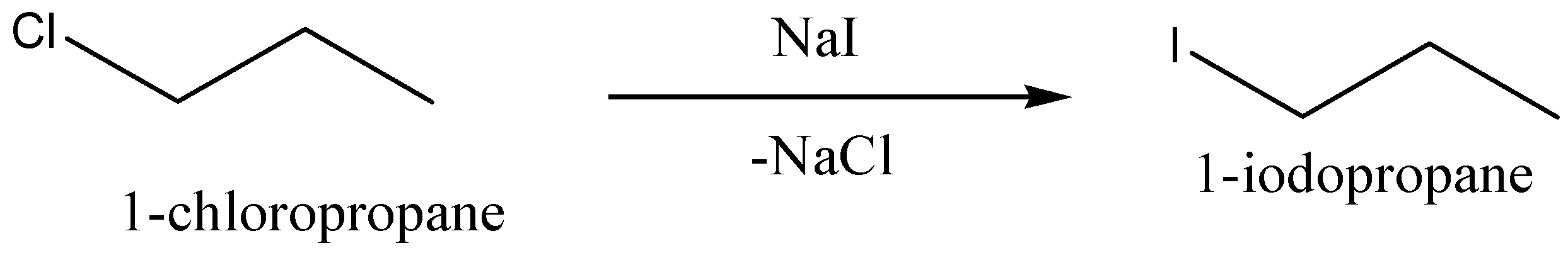
Now we can discuss the reaction of 1−chloropropane with potassium cyanide,KCN, to give butane nitrile (chemical formula: C4H7N). And potassium chloride,KCl, is eliminated.
We can write the chemical equation for this chemical reaction as,
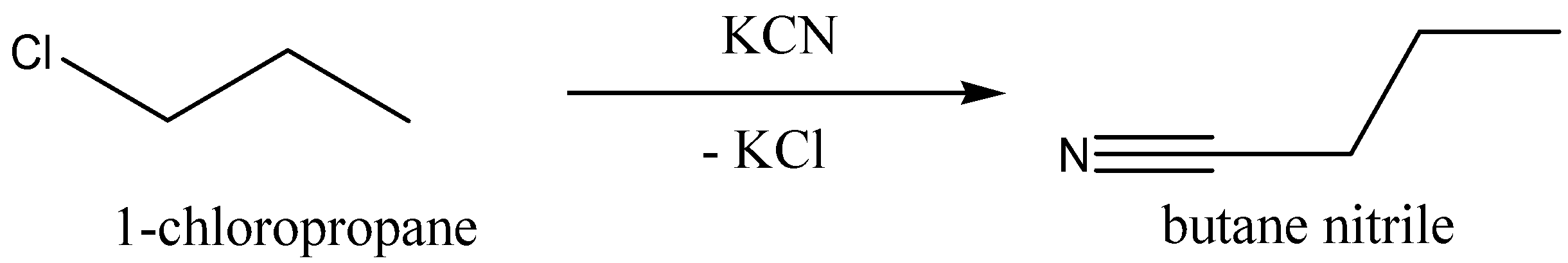
Now we can discuss the conversion of 1−chloropropane into Propyl ethanoate.
When the 1−chloropropane is reacted with alcoholic potassium hydroxide,KOH, to give the product Propan−1−ol, the chemical formula for Propan−1−ol is C3H7OH. And potassium chloride,KCl, is the byproduct for this reaction. Then Propan−1−ol is treated with propionic acid, chemical formula-C3H6O2, to give the corresponding product Propyl ethanoate (chemical formula:C5H10O2), and eliminates water molecule.
We can write the chemical reaction for Conversion of 1−chloropropane into Propyl ethanoate as,
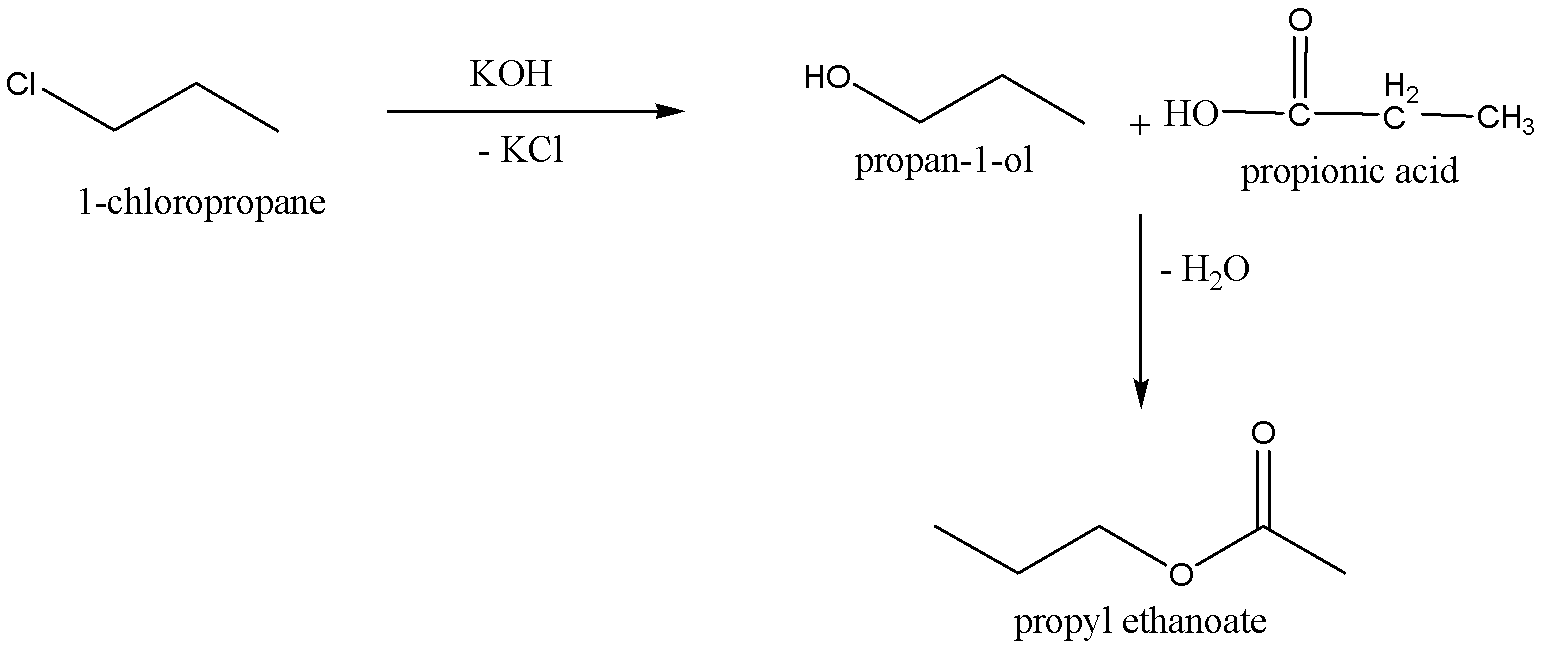
Therefore, the option A and B is correct.
Note: All the above three conversions (options a, b and d) contain the same carbon atoms on both sides, reactant sides and product sides. But in option (c), Conversion of 1−chloropropaneinto Butane nitrile, that butane nitrile containing one carbon atom more than the reactant,1−chloropropane.
1−chloropropane is used in pharmaceutical intermediate for n−propylamine and solvent for making other chemicals which is also used in pesticides.
