Question
Question: Conversion of chlorobenzene to phenol involves A) Electrophilic substitution B) Nucleophilic su...
Conversion of chlorobenzene to phenol involves
A) Electrophilic substitution
B) Nucleophilic substitution
C) Free radical substitution
D) Electrophilic addition
Solution
The answer to this question is based upon the general concept of organic chemistry that deals with the named reaction called Dow's process. This conversion involves reagent as a base which is an aromatic substitution reaction and the answer lies here.
Complete step by step answer:
We have studied in the lower classes of organic chemistry about various named reactions and also the reagents used for those.
We shall now see what Dow's process means and deduce the required answer.
- Dow’s process is actually the electrolytic method of bromine extraction from brine but this process also applies to the preparation of phenol.
- In this process of preparation of phenol, the starting material used is chloro benzene.
- The hydrolysis of chloro benzene in the presence of a base that is sodium hydroxide yields the compound phenol.
- At higher temperature about 3500C and high pressure of about 300bar or also molten sodium hydroxide at 3500C is used to convert chloro benzene to phenol.
- In this reaction, the intermediate formed is benzyne that is unstable and hence on further reaction with base gives sodium phenoxide which when treated with acid yields phenol.
The reaction mechanism is as shown below:
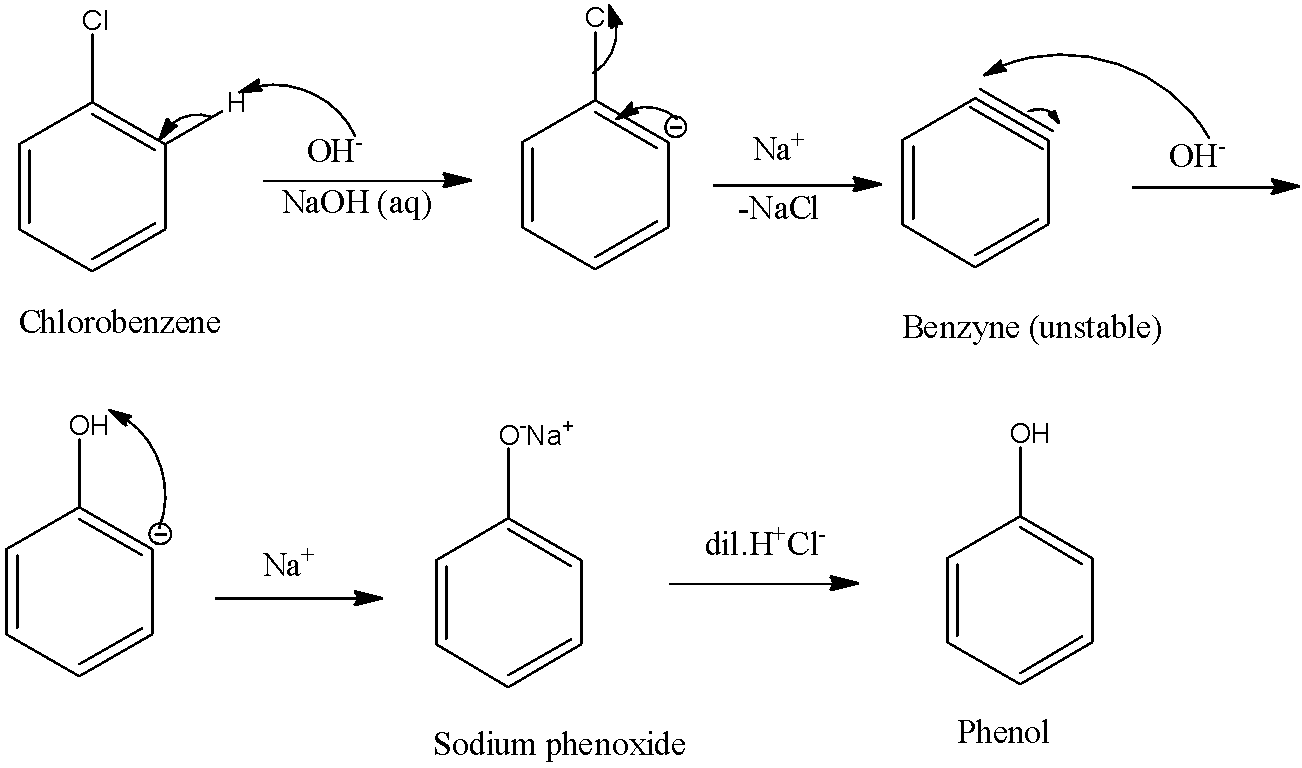
Therefore, this is a substitution reaction.
Now, since the substituted group (−OH) and the leaving group (Cl−) are both nucleophiles, this particular reaction can be called the nucleophilic substitution reaction. So the correct answer is “B”:
Note: Note that substitution reaction and addition reactions are two different terms where the addition reaction takes place when two or more reactants combine to form a single product and this will contain all the atoms which were present in the reactants whereas substitution reaction takes place when there is an exchange of elements in the reactants With each other. Do not be confused about this fact.
