Question
Question: Conversion from Benzene to 1, 4-dichorobenzene ....
Conversion from Benzene to 1, 4-dichorobenzene .
Solution
Direct conversion of benzene to 1, 4-dichlorobenzene is not easy so first, the benzene is converted into chlorobenzene and then this chlorobenzene is converted into 1, 4-dichlorobenzene.
Complete answer:
Benzene is an aromatic compound of six carbon atoms that form a ring structure and contains three double bonds. The chemical formula of benzene is C6H6. The structure of benzene is given below:
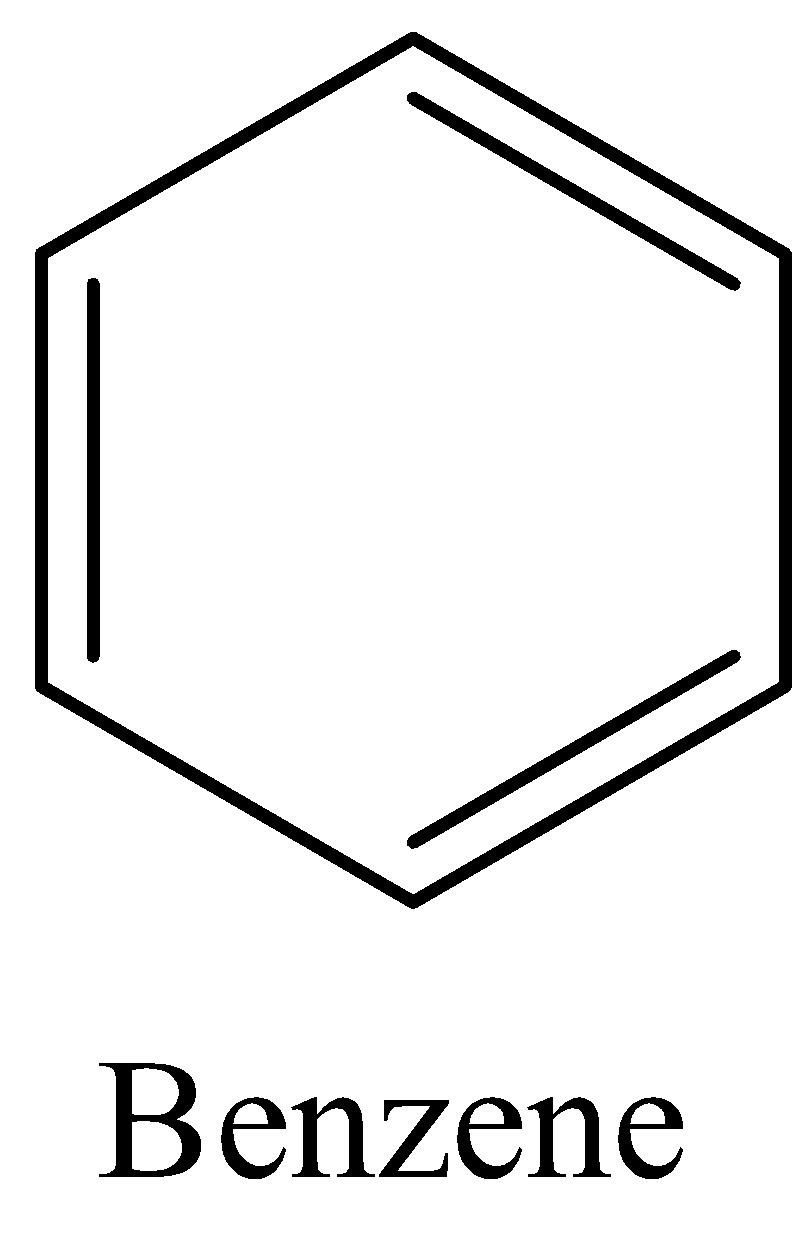
1, 4-Dichlorobenzene is an aromatic compound in which there are two chlorine atoms present in the benzene ring i.e., at 1st position and 4th position of the carbon atom. The chemical formula of 1, 4-Dichlorobenzene is C6H4Cl2. 1, 4-Dichlorobenzene is also known as p-Dichlorobenzene. The structure of 1, 4-Dichlorobenzene is given below:
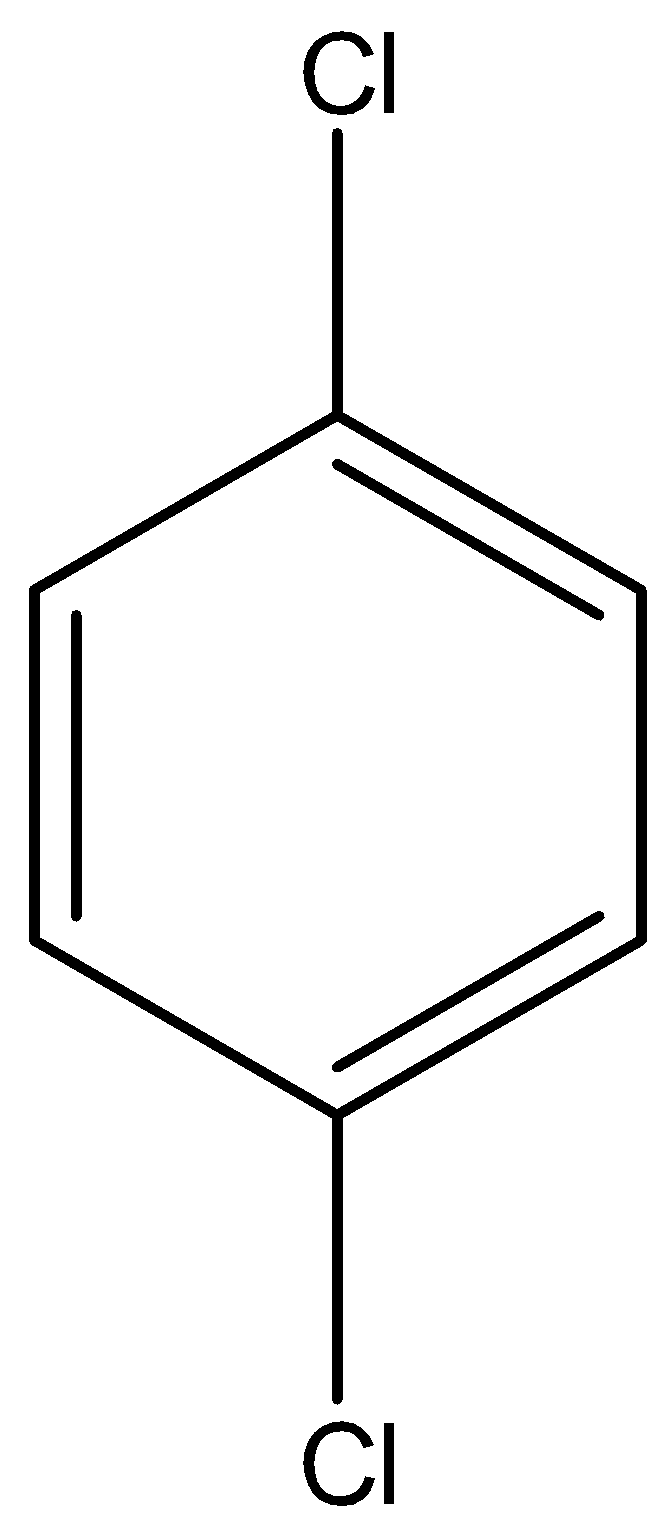
Benzene cannot be directly converted into 1, 4-Dichlorobenzene. So first, we have to convert the benzene to chlorobenzene and then this chlorobenzene is converted into 1, 4-Dichlorobenzene.
So first the benzene is converted into chlorobenzene by reacting to the benzene with chlorine and ferric chloride. So the reaction is given below:
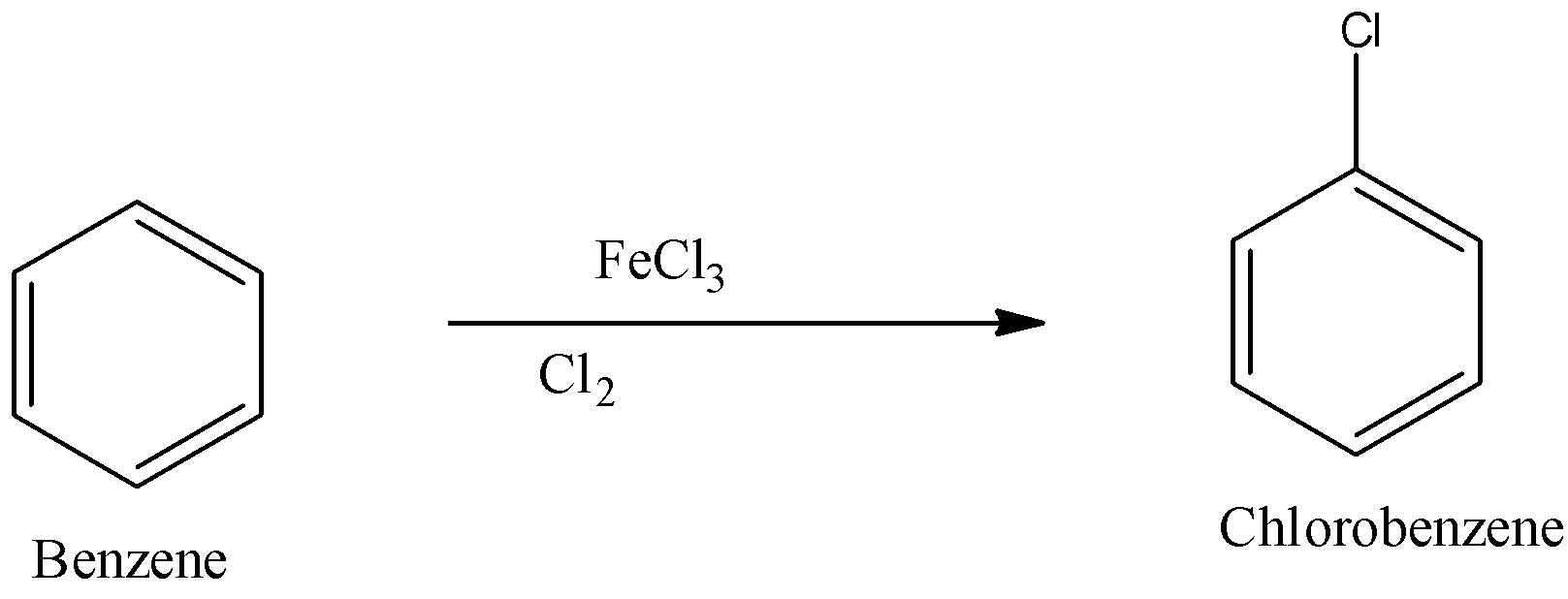
Now this chlorobenzene can be converted into 1, 4-Dichlorobenzene or p-dichlorobenzene. When chlorobenzene reacts with chlorine and ferric chloride there is a formation of 1, 2- Dichlorobenzene or o- Dichlorobenzene and 1, 4- Dichlorobenzene or p- Dichlorobenzene. The reaction is given below:
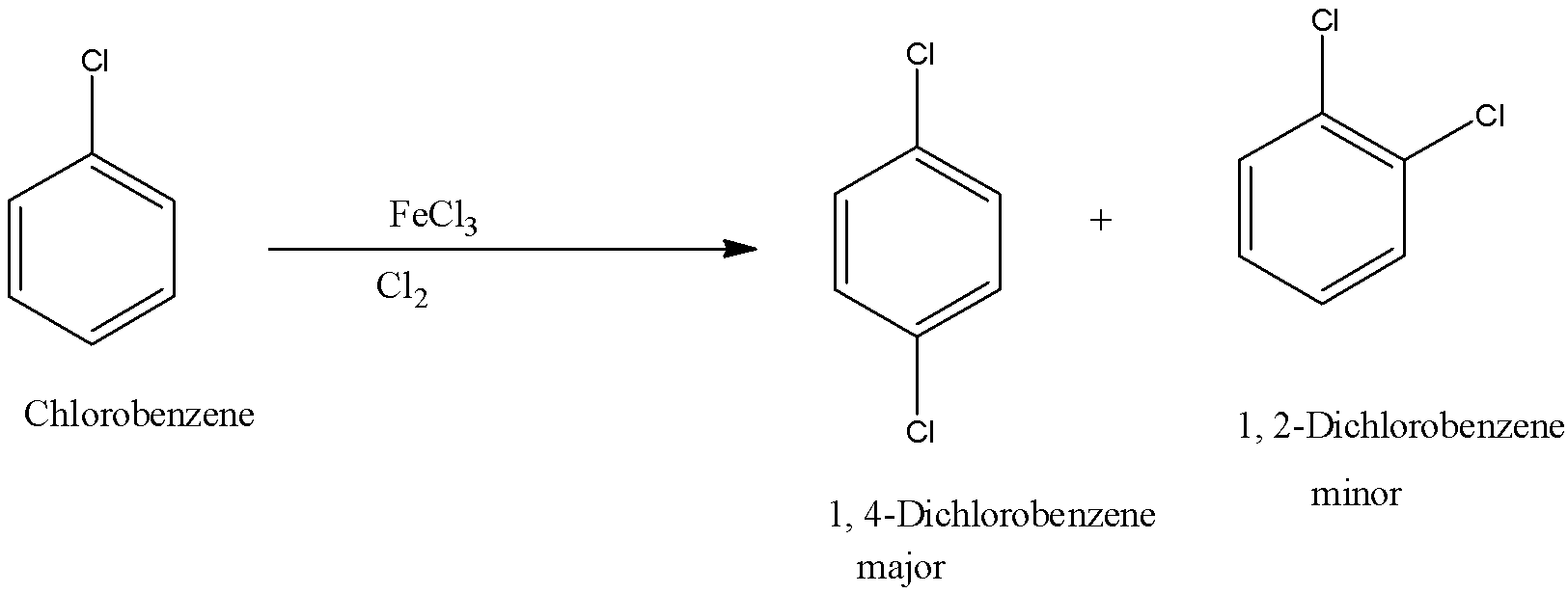
In this reaction 1, 4- Dichlorobenzene is the major product, and 1, 2- Dichlorobenzene is the minor product.
So these steps must be followed to convert benzene to 1, 4- Dichlorobenzene.
Note:
1, 4- Dichlorobenzene is the major product because of the symmetry in the molecule and it fits into the lattice and 1, 2- Dichlorobenzene doesn't have a symmetric molecule so it is the minor product.
