Question
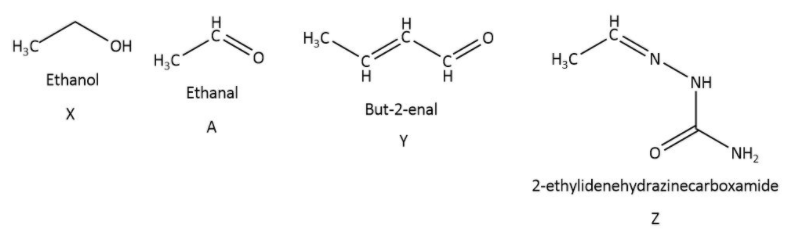
Question: Consider the reactions: Identify A, X, Y and Z. 
A. A-Ethanol, X-Acetaldehyde, Y-Butanone, Z-Hydrazone
B. A-methoxymethane, X- Ethanoic acid, Y-Acetate ion, Z-Hydrazine
C. A-methoxymethane, X- Ethanol, Y-Ethanoic acid, Z-Semicarbazide
D. A-Ethanal, X-Ethanol, Y- but−2−enal, Z-Semicarbazone
Solution
In the above conversion, the molecular formula is given which will give us the idea that the molecule with formula C2H6O is having hydroxyl group. Further, the reagents used can give the idea that molecule A can be a carbonyl group.
Complete answer:
Here from the given question as we know that the silver mirror test is given by the aldehyde and not by ketone hence, the product A in the answer has to be aldehyde. We can eliminate options A, B and C and check the option D for X, Y and Z.
X has to be ethanol because C2H4O2 is the molecular formula for ethanoic acid and the formula for acetaldehyde is C2H4O which does not matches with the question which ask the reaction for C2H6O.
Hence, X will be alcohol known as ethanol with structural formula as shown in the figure given below.
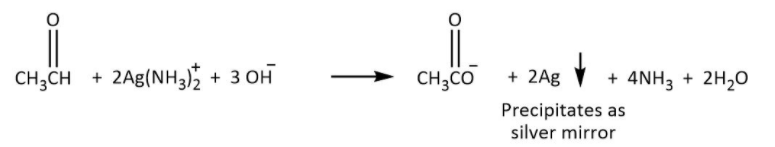
Tollen’s test: It is the test used to differentiate between the ketone and aldehydes. The use of reagent known as ammoniacal silver nitrate is used to detect the Aldehydes. The use of this reagent is for oxidizing aldehyde to form carboxylic acid and the reagent will be reduced to silver. As the aldehyde produced free silver due to reduction of ammoniacal silver nitrate the reaction is also known as Silver mirror test for aldehyde. The number of carbons will remain the same during the reaction.
Hence, the structure of product A which is ethanal will be as shown in the figure above, then it is possible that it will give a silver mirror test. Given by the following reaction in which aldehyde is converted to carboxylic acid.
Reaction of ethanal in the presence of hydroxyl ion will give us but−2−enal because that reaction is nothing but aldol condensation. In aldol condensation the two molecules of the aldehyde will combine in presence of base to produce β−hydroxyaldehydewhich is known as aldol hence, the reaction is aldol condensation reaction. Here as we can see the molecule is ethanal or acetaldehyde which will condense in presence of base like sodium hydroxide to produce 3−hydroxybutanal it will further dehydrate in presence of acid to give but−2−enal.
Product Z is 2−ethylidenehydrazinecarboxamide or semicarbazone which is nothing butα−βunsaturatedaldehyde formed due to conversion of aldehyde to semicarbazone derivative in presence of semicarbazide because it contain nitrogen as nucleophile.
**Hence, the correct answer is (D) A-Ethanal, X-Ethanol, Y-But-2-enal, Z-Semicarbazone
Note: **
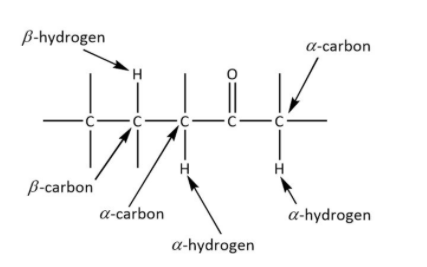
The recognition of alpha and beta carbon is important to decide the product. The given figure shows the correct alpha and beta carbon.