Question
Question: Consider the reactions given below and identify A and B: \({\text{A}}\xleftarrow[{{\text{C}}{{\tex...
Consider the reactions given below and identify A and B:
ABD3/THFCH3COOHPenteneBD3/THFCH3COODB
A. A = 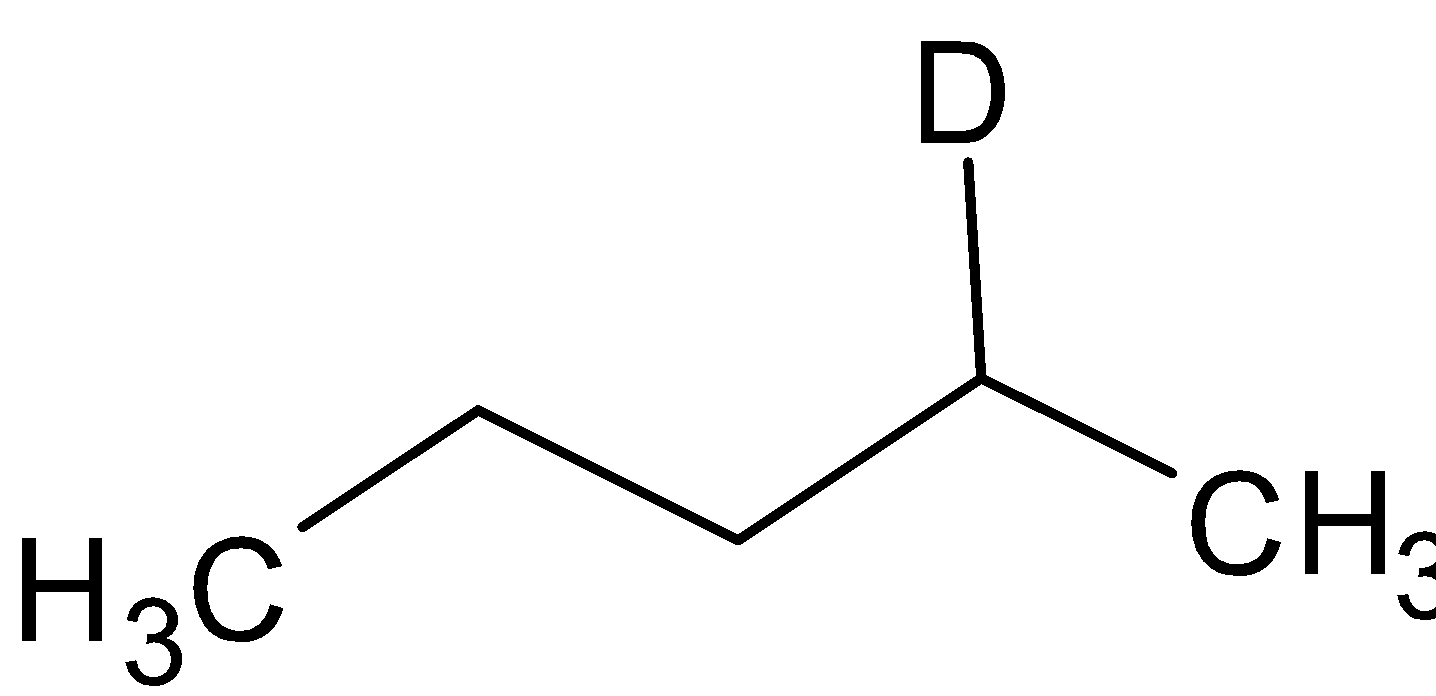 B=
B=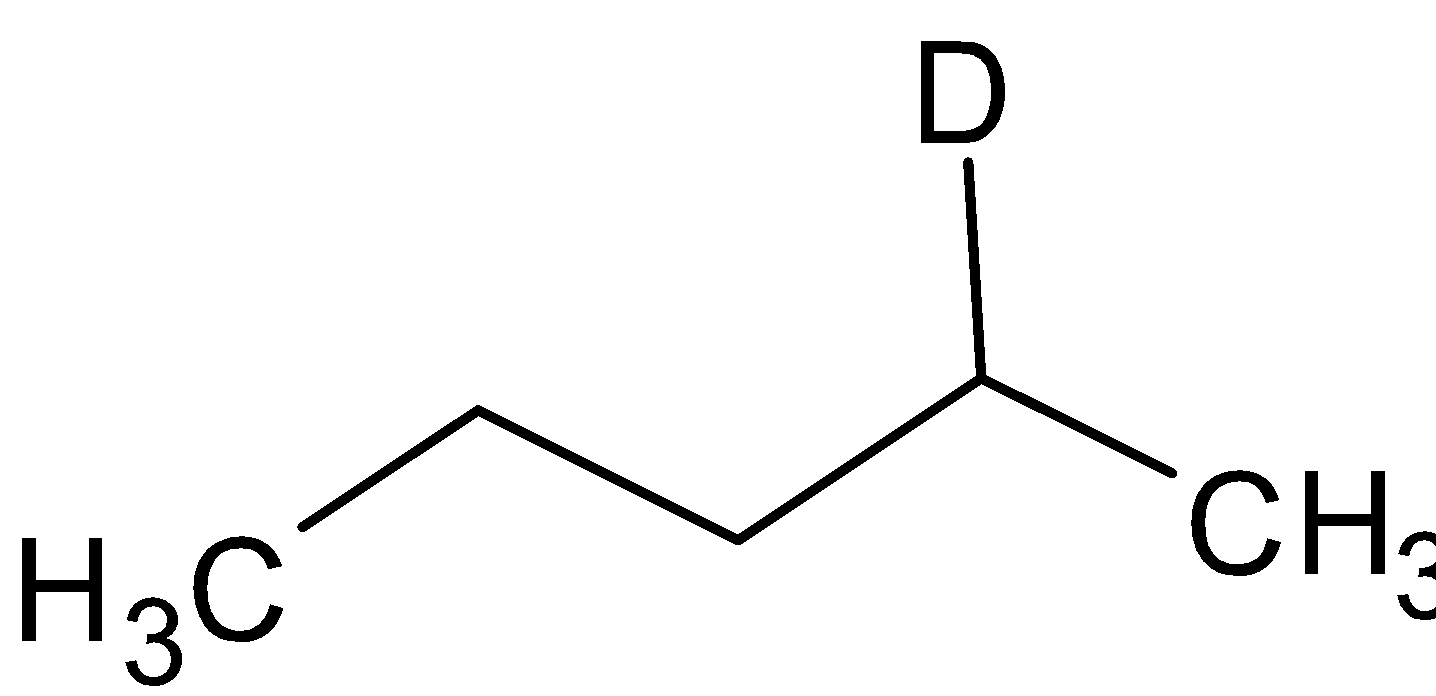
B. A = 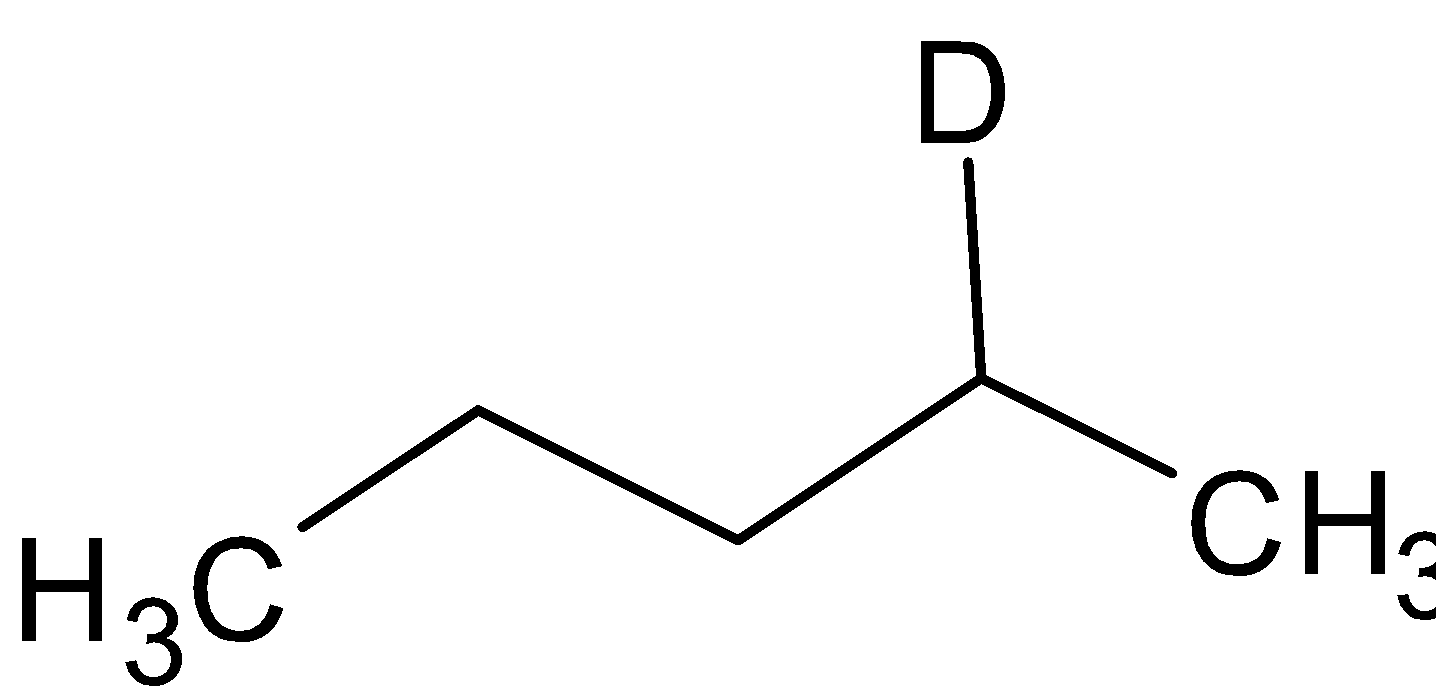 B=
B= 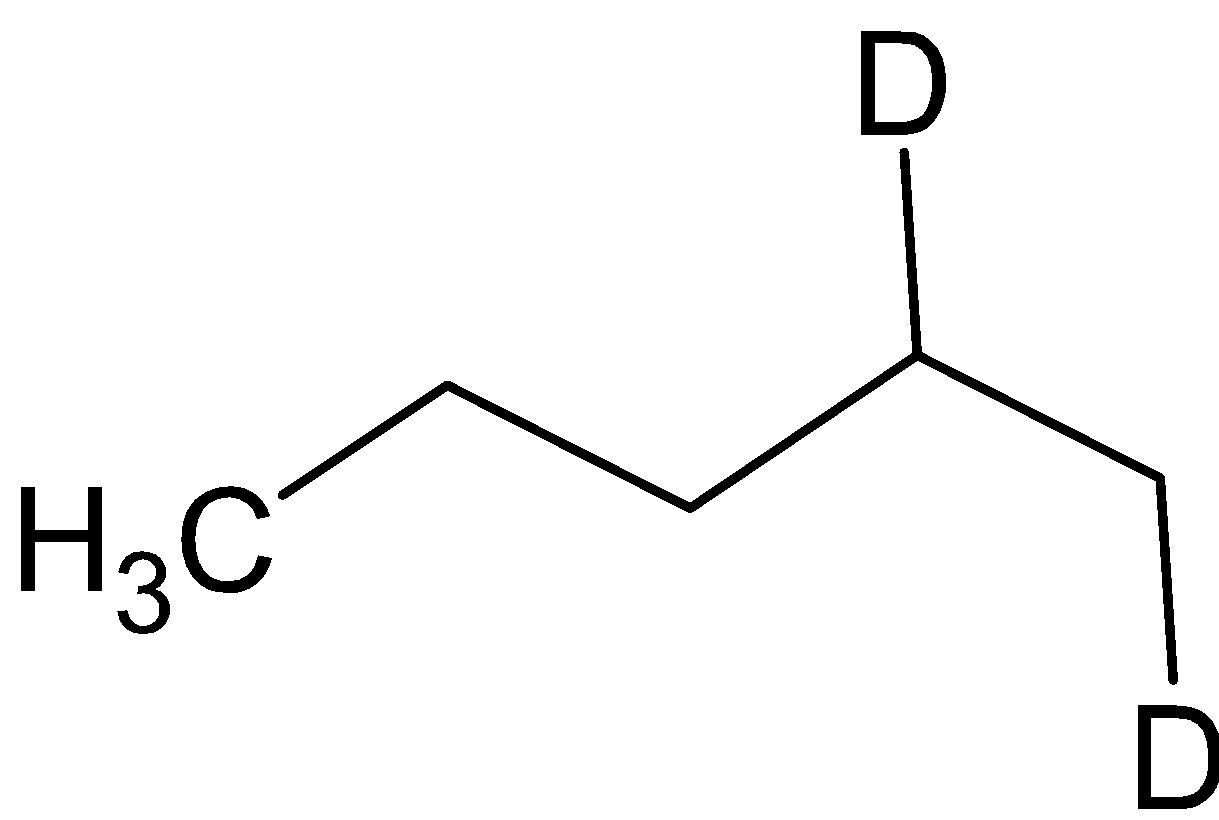
C. A = 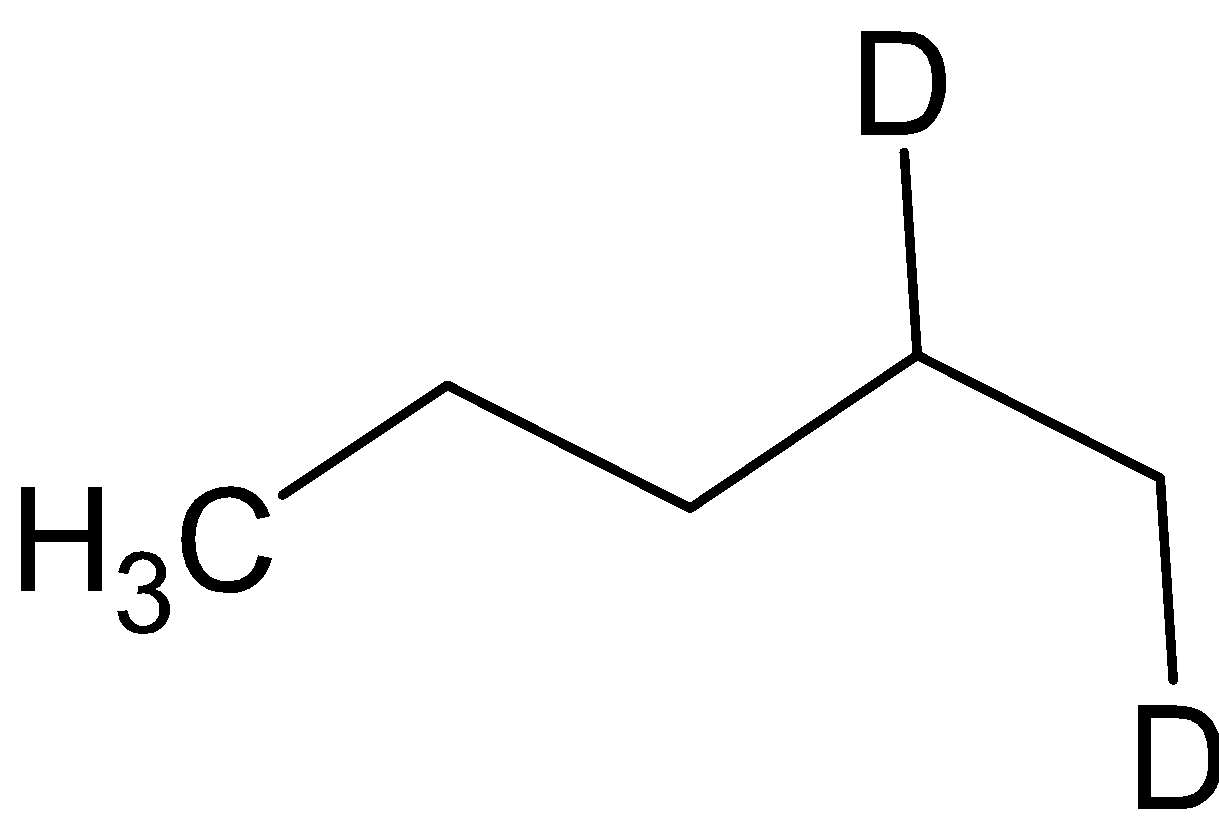 B=
B= 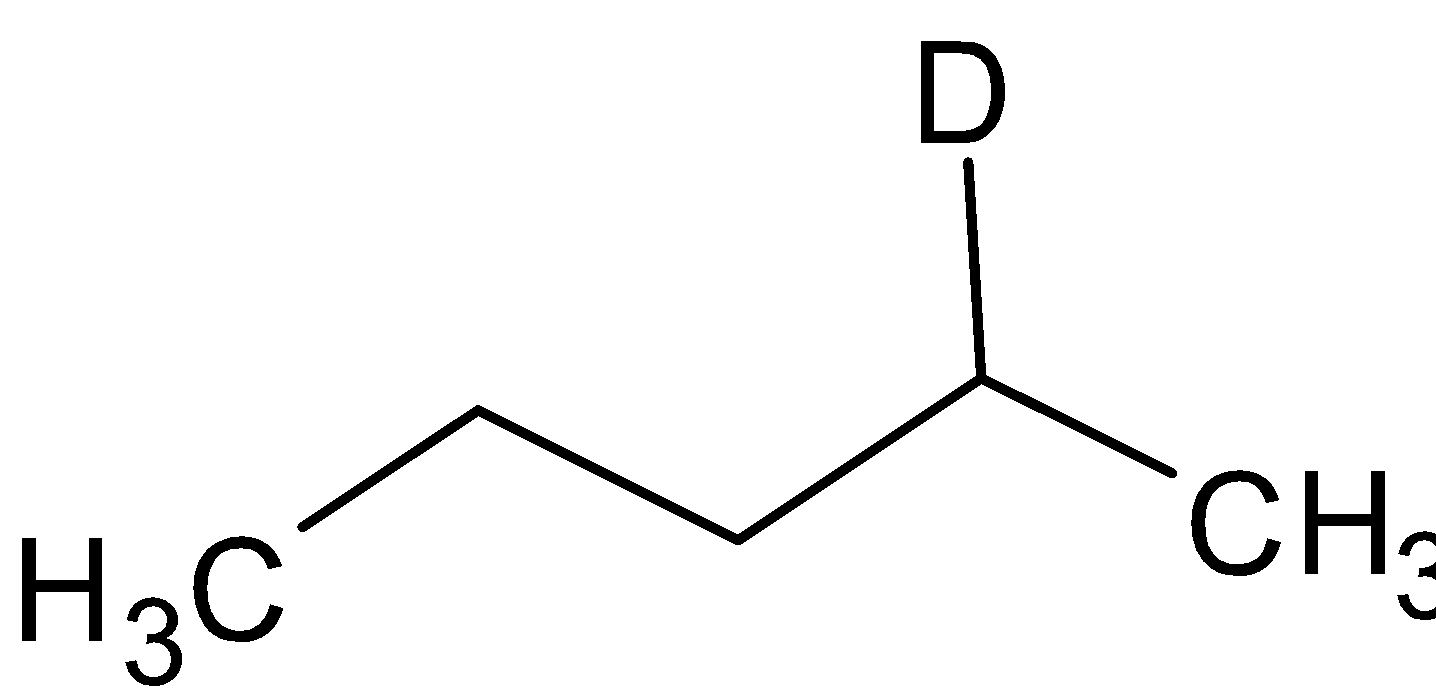
D. A = 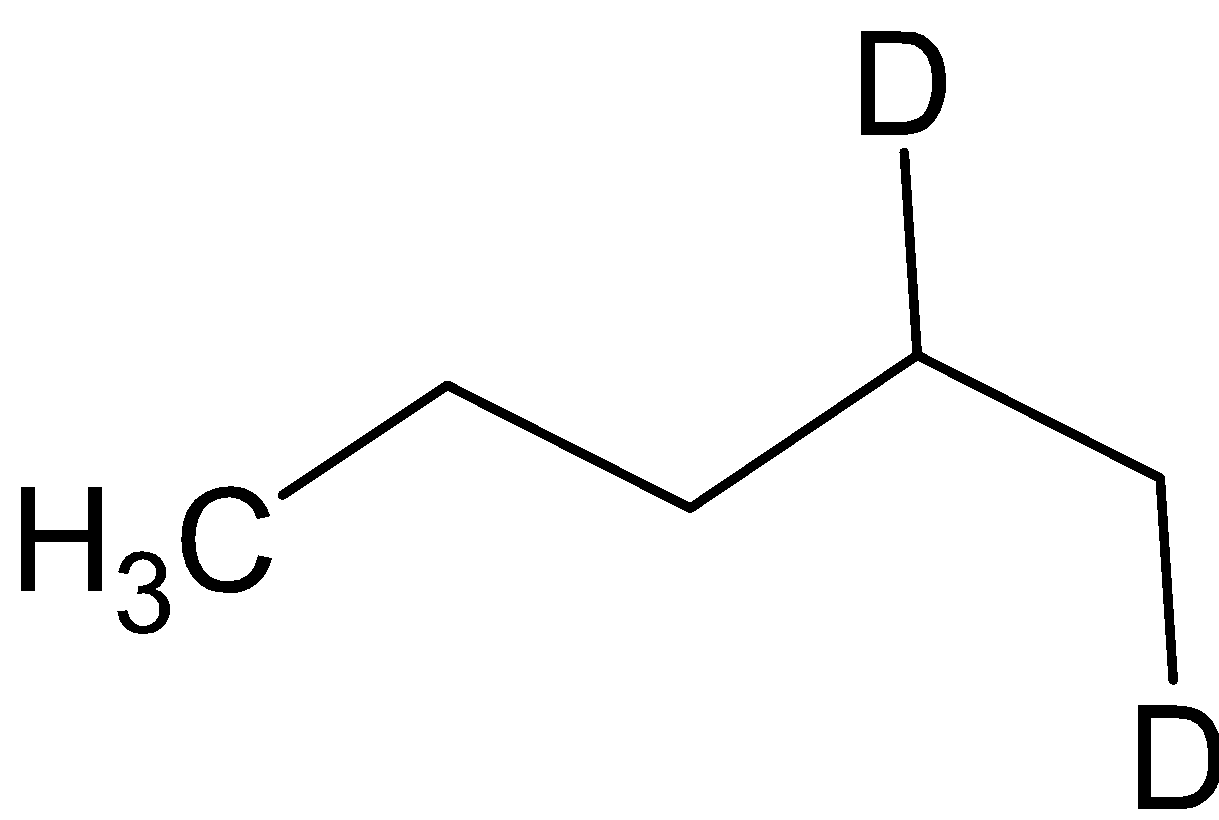 B=
B=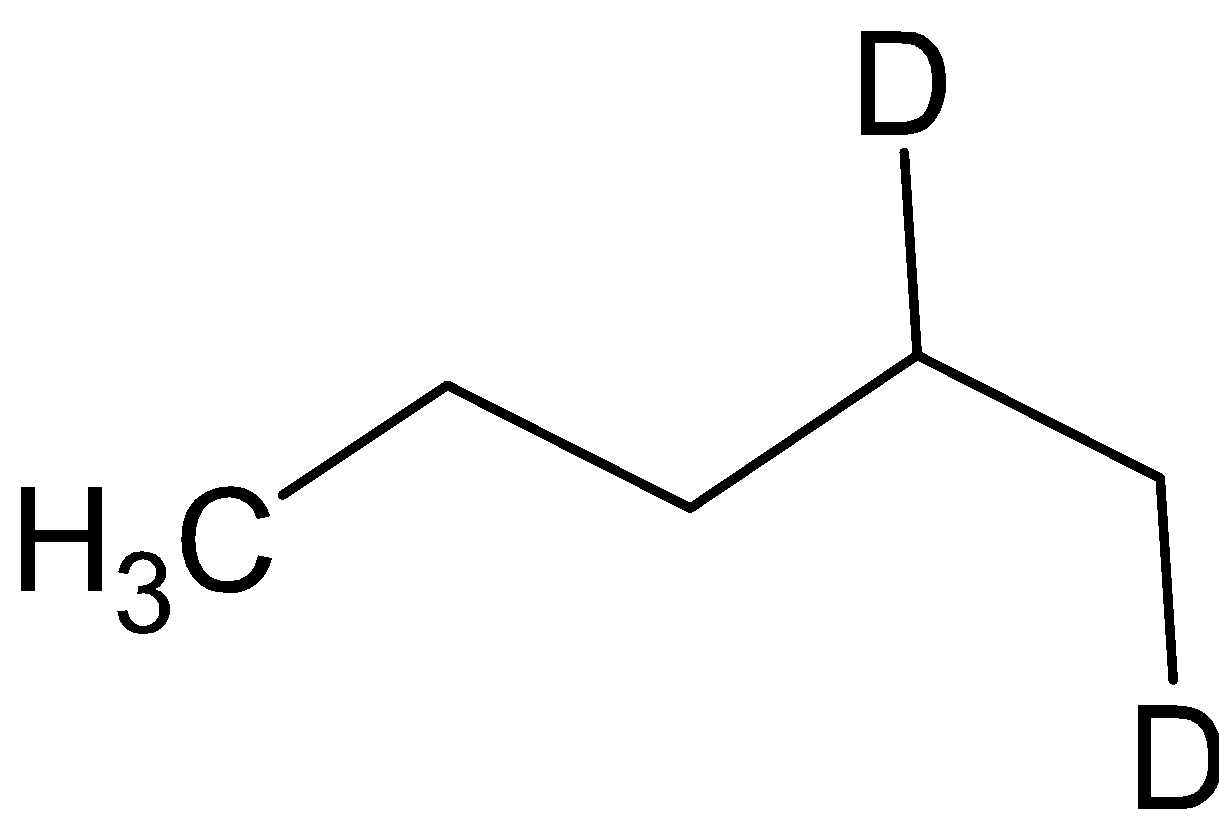
Solution
Borane dissolved in THF undergoes additional reactions rapidly with most alkenes. This reaction is called hydroboration. Hydroboration produces an organoborane. This is a very useful intermediate in organic synthesis.
Complete step by step answer:
Alkenes are also called olefins. They contain at least one carbon-carbon double bond. In such compounds, carbon is sp2 hybridized.
One of the major reactions of alkenes is hydroboration reaction. It is the addition of hydrogen-boron bonds to carbon-carbon, carbon-nitrogen, and carbon-oxygen double bonds, as well as triple bonds. This chemical reaction is used for organic synthesis of compounds.
Here, instead of BH3, BD3is used. Hydrogen is substituted with deuterium. Generally they are Lewis acid since they are electron deficient. Borane is added to alkene to give organoborane. The reaction is given below:
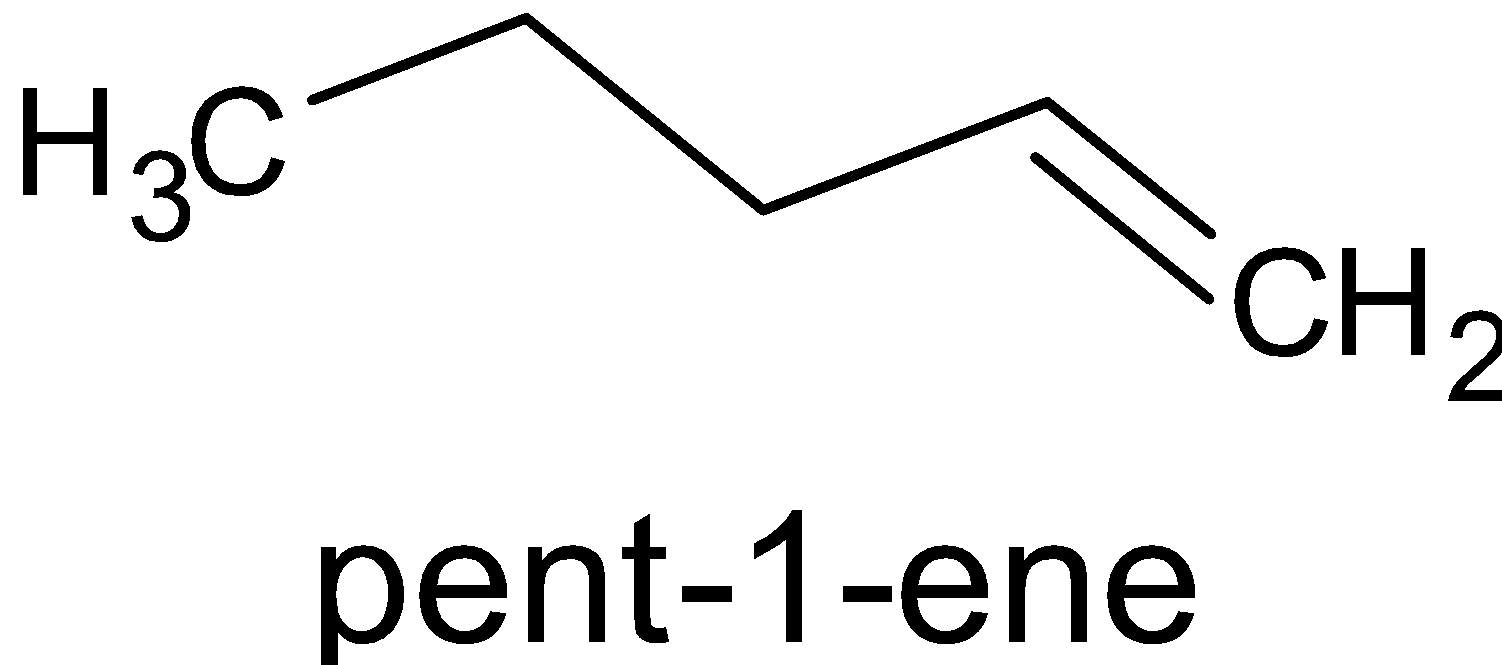 BD3/THF
BD3/THF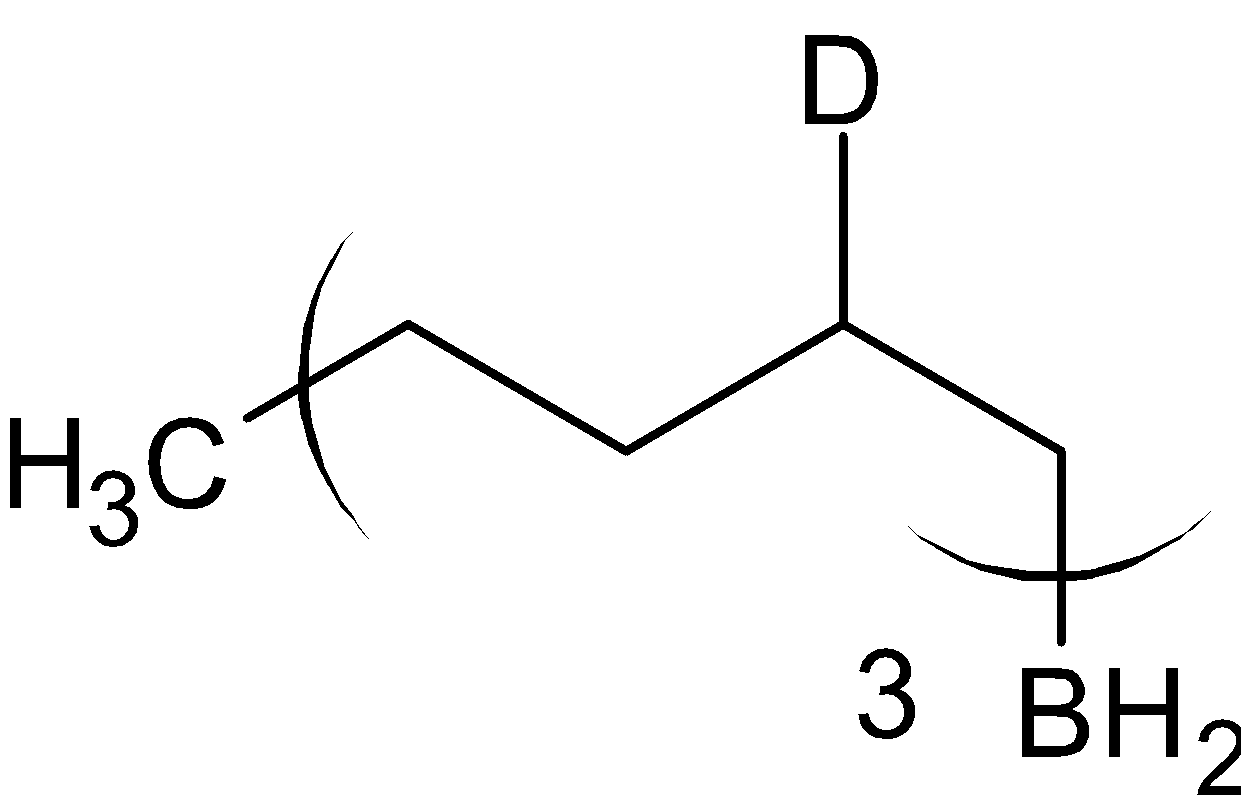
The product formed is called organoborane. This is now divided into two reactions, with acetic acid, CH3COOH and with deuterated acetic acid, CH3COOD .
When organoborane is reacted with acetic acid, it substitutes borane with methyl group from acetic acid. When the organoboron is reacted with deuterated acetic acid, deuterium is substituted in the place of borane. The reaction is given below:
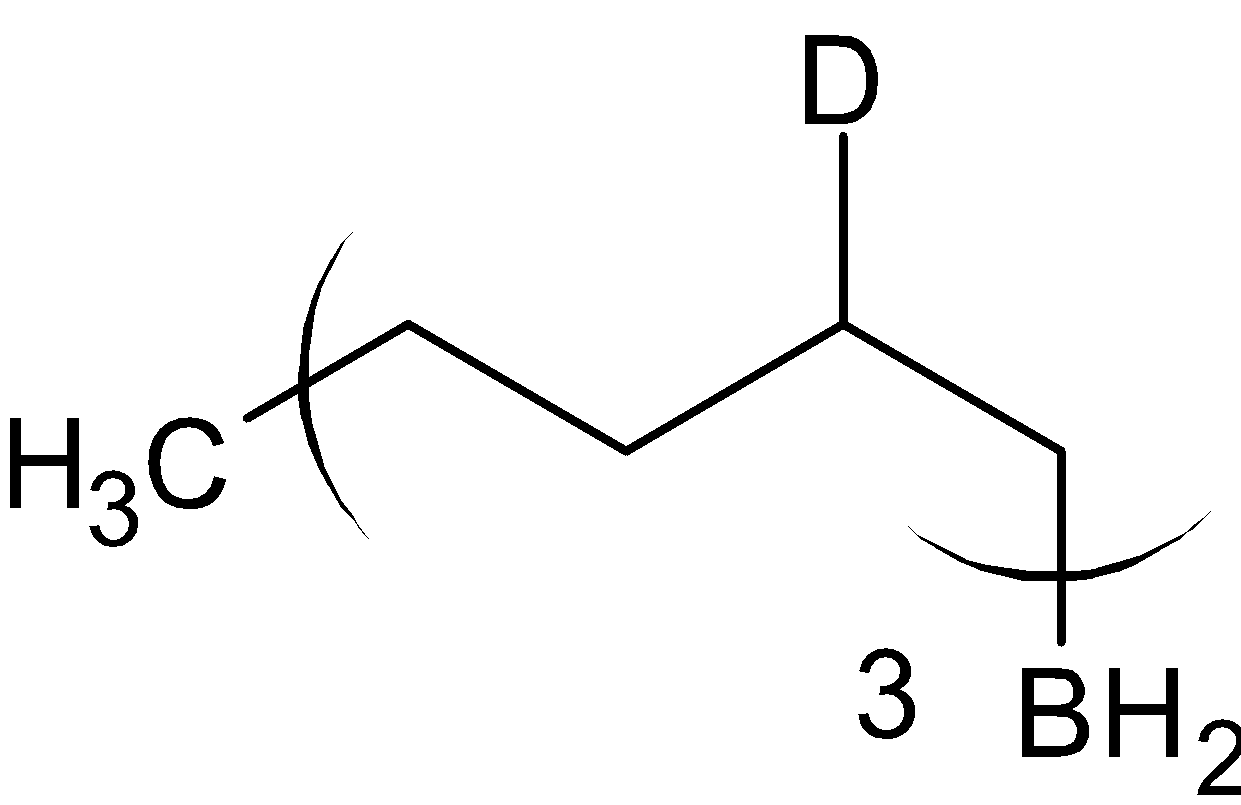 CH3COOH
CH3COOH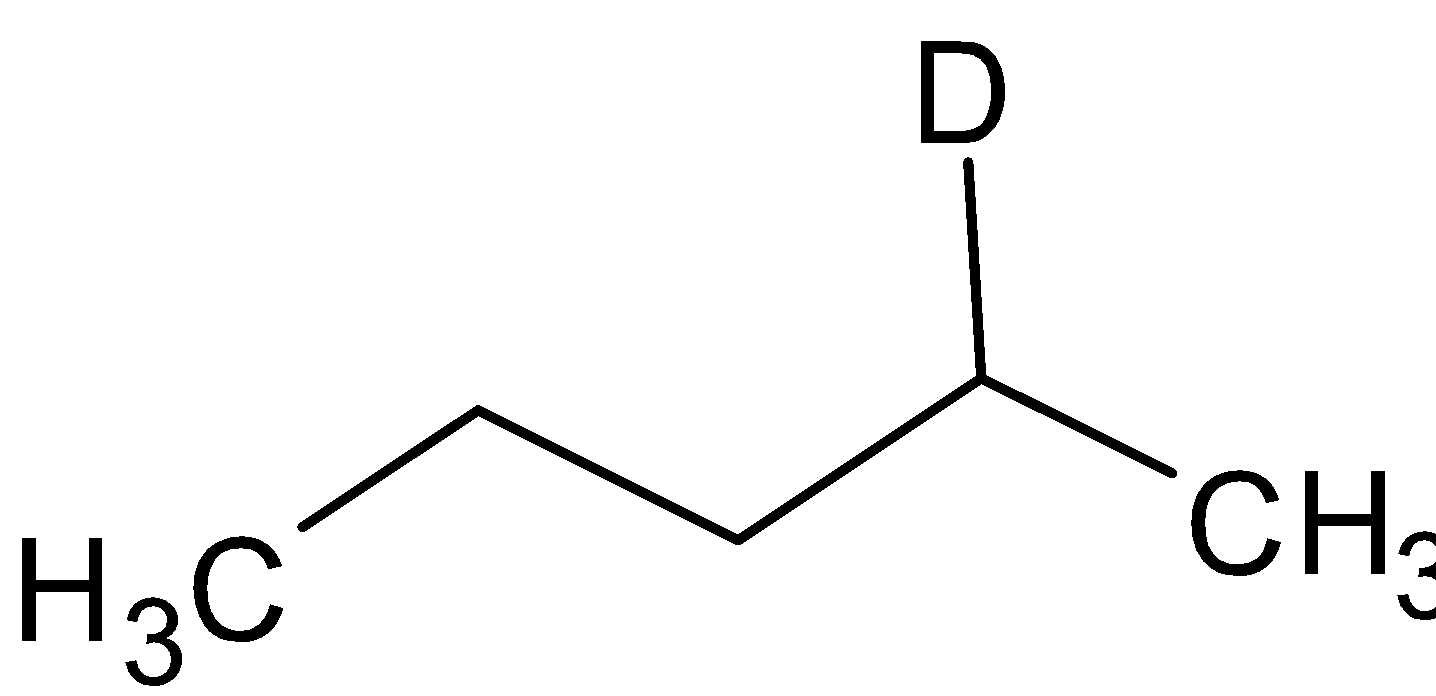
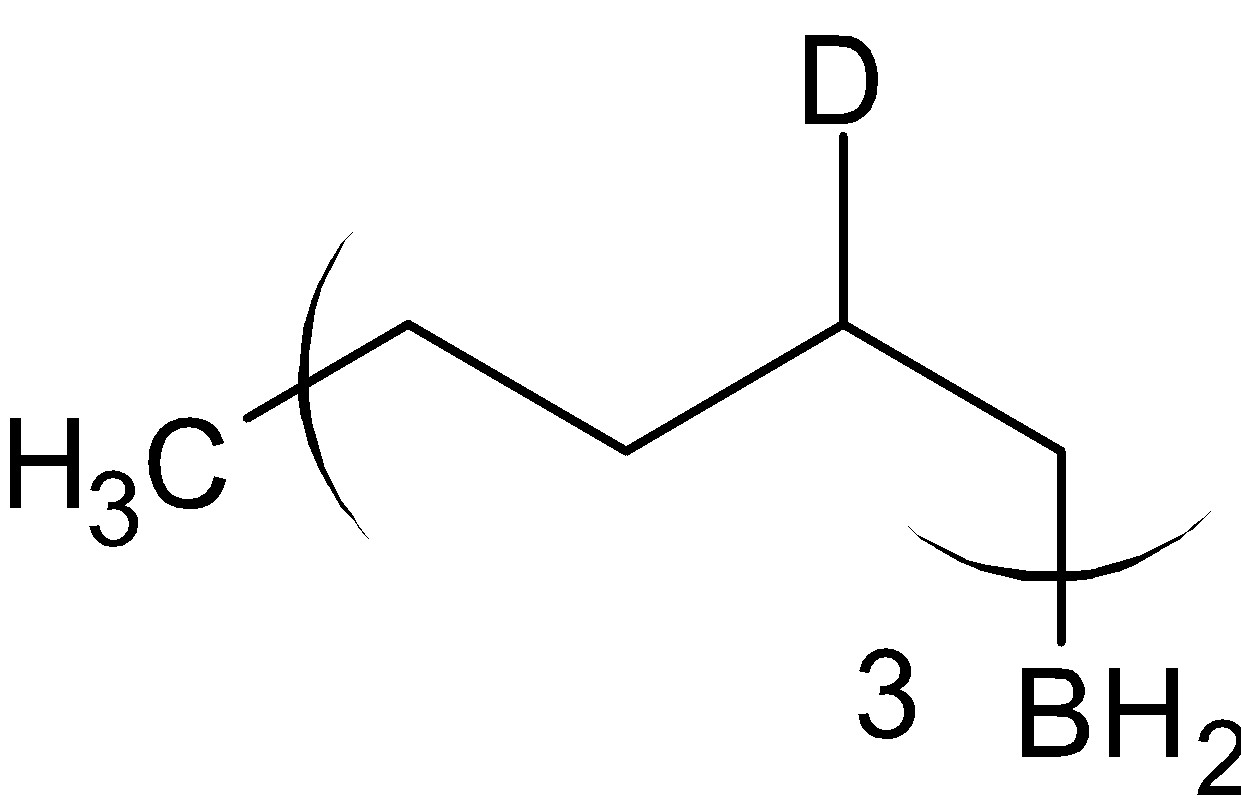 CH3COOD
CH3COOD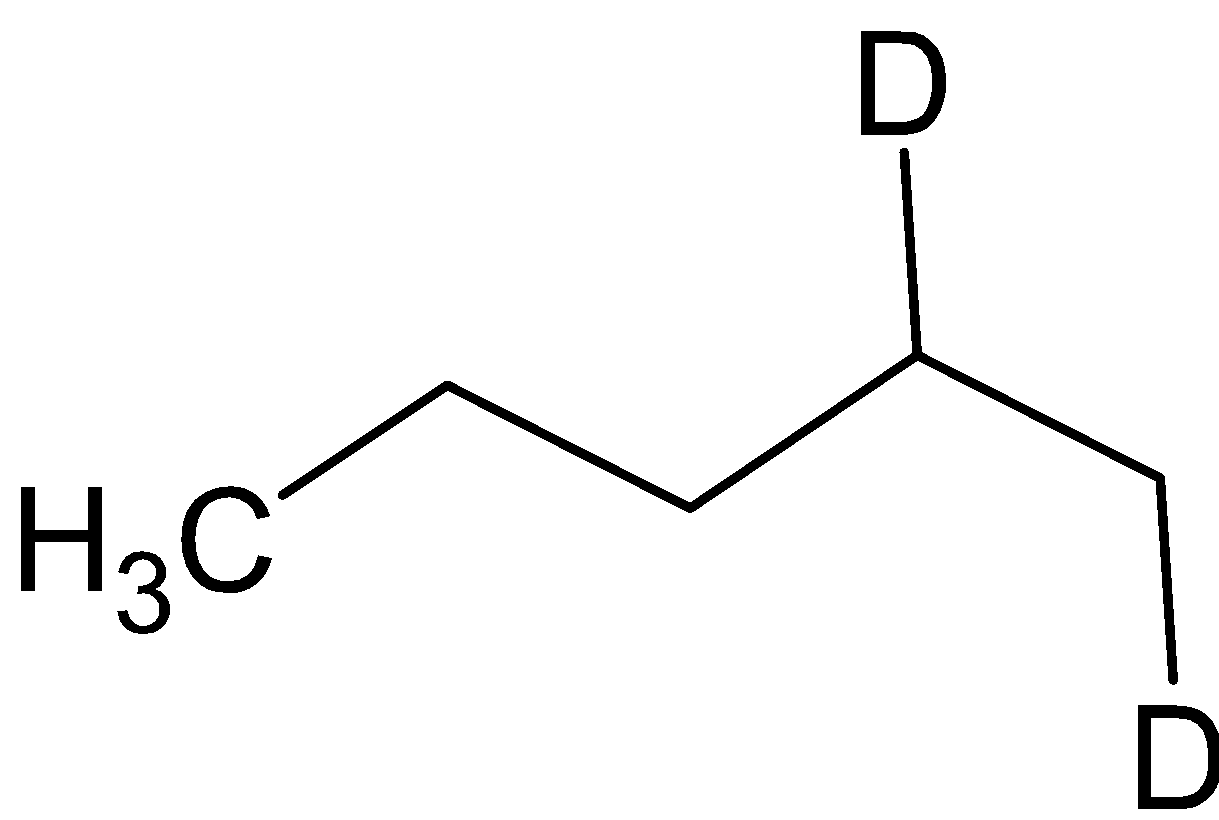
Thus the compound A is 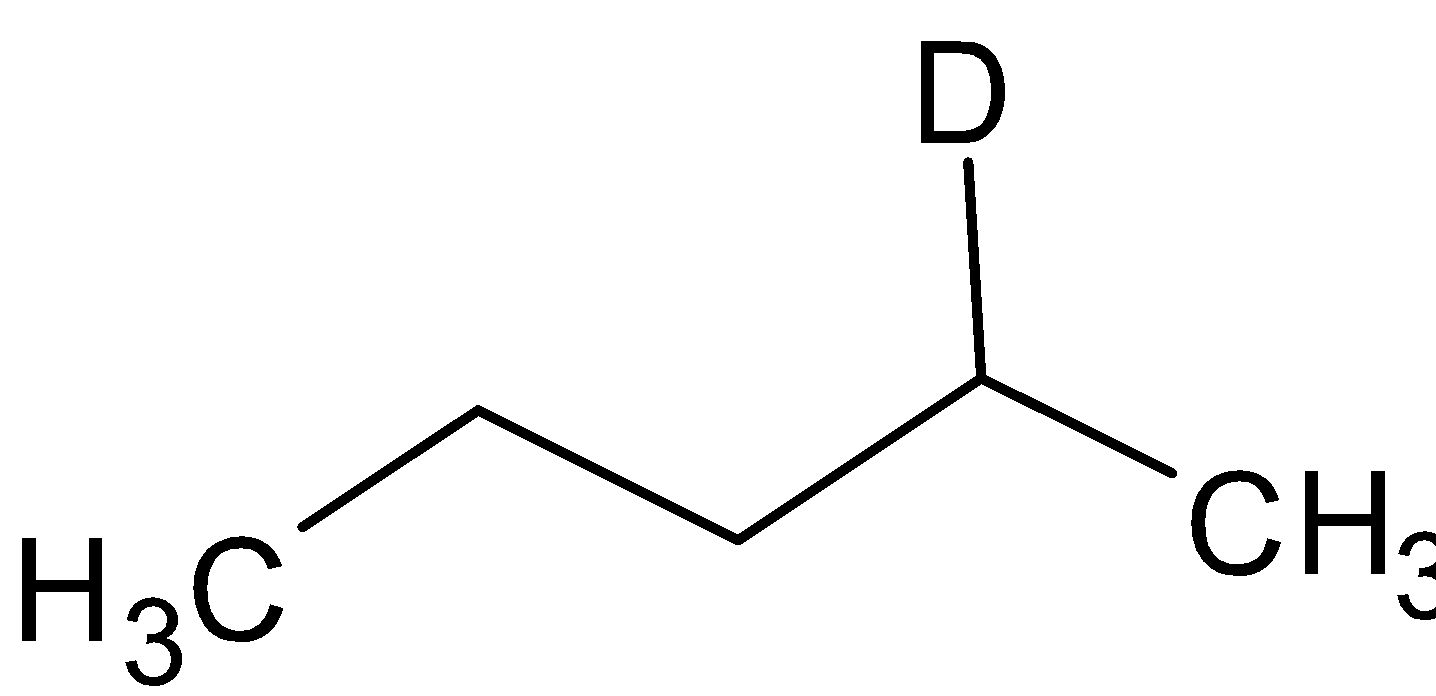 and the compound B is
and the compound B is 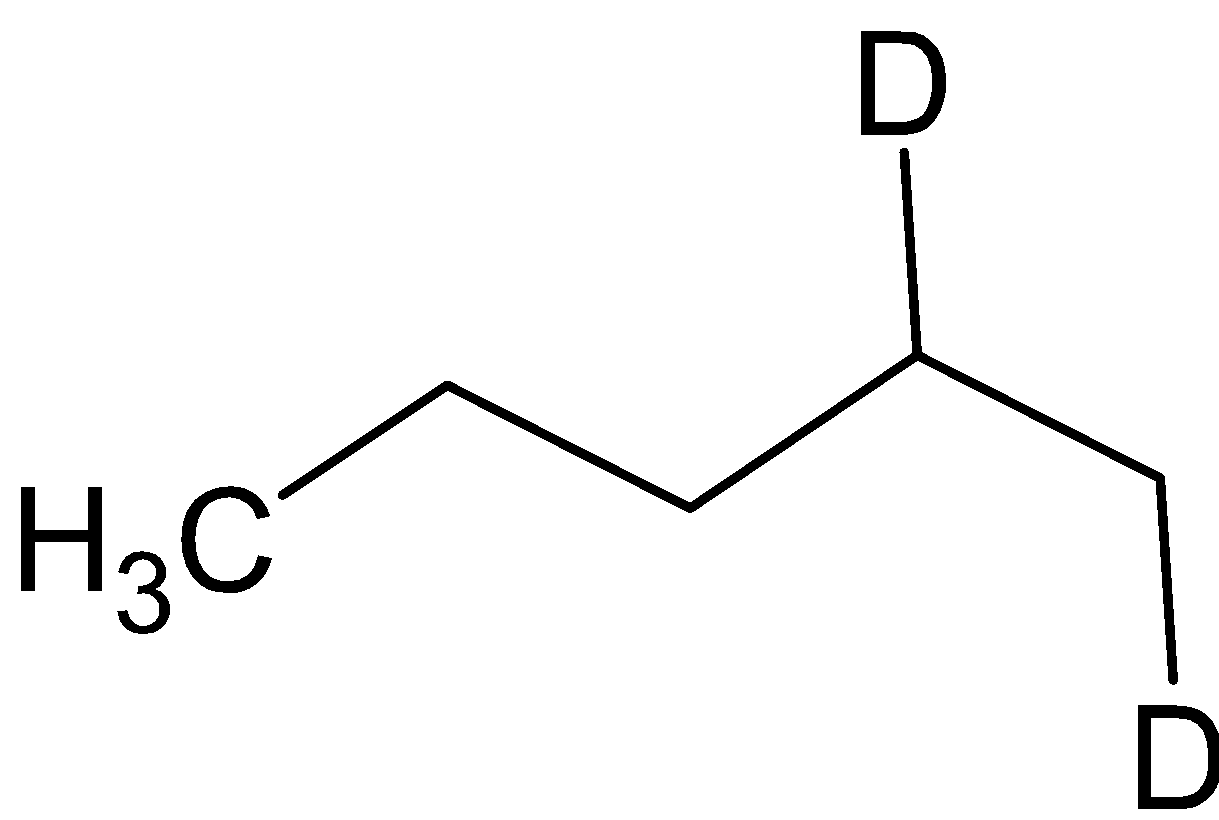
Hence option B is correct.
Additional information:
BH3.THF is the most commonly used form of borane. Hydroboration steps add the hydrogen and the boron to the same side of the double bond.
Note:
Deuterated borane adds to the double bond in a single step, with boron adding to the less substituted carbon and hydrogen adding to the more highly substituted carbon. This orientation places the partial positive charge in the transition state on the more highly substituted carbon atom.
