Question
Question: Consider the reaction given below :- Select the correct statement(s)...
Consider the reaction given below :-
Select the correct statement(s)
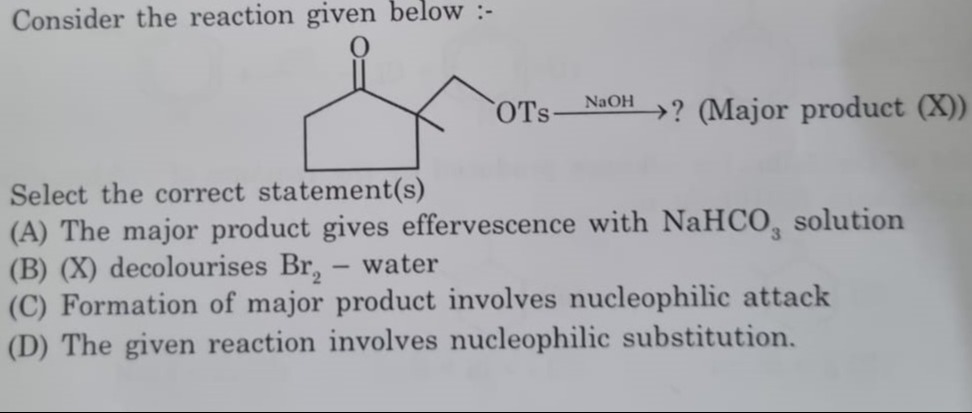
The major product gives effervescence with NaHCO3 solution
(X) decolourises Br2 - water
Formation of major product involves nucleophilic attack
The given reaction involves nucleophilic substitution.
(C), (D)
Solution
The reactant is 2-methyl-2-(1-(tosyloxy)ethyl)cyclopentanone. The reaction is with NaOH, a strong base and nucleophile. OTs is a good leaving group. The carbon bearing the OTs group (C6 in our numbering) is a secondary carbon. Beta carbons are C2 and the methyl carbon attached to C6. C2 is a quaternary carbon, so it has no beta-hydrogens. The methyl carbon on C6 has hydrogens. Elimination from the methyl group on C6 would lead to a double bond between C6 and the methyl carbon, which is not a typical elimination pathway.
However, consider the possibility of intramolecular reaction. The alpha hydrogens on C5 are acidic. Deprotonation by NaOH can form an enolate at C5. This enolate can act as a nucleophile. The carbon with the leaving group OTs is C6. The distance between the enolate carbon C5 and C6 is suitable for intramolecular cyclization. Attack of the enolate carbon C5 on C6 would form a 5-membered ring. Let's draw the structure and the reaction.
The reactant is 2-methyl-2-(1-(tosyloxy)ethyl)cyclopentanone. Let's number the carbons: C1 (ketone), C2 (adjacent to ketone, with CH3 and side chain), C3, C4, C5 (adjacent to ketone). Side chain is -C6H(CH3)-OTs attached to C2.
Deprotonation at C5 by NaOH forms an enolate. The enolate carbon C5 is nucleophilic. The leaving group OTs is on C6. Intramolecular nucleophilic attack of C5 on C6 will form a new C-C bond between C5 and C6, and the OTs group will leave. This reaction forms a bicyclo[3.2.1]octane ring system with a ketone group.
The product is 2,6-dimethylbicyclo[3.2.1]octan-8-one.
Let's evaluate the statements. (A) The major product gives effervescence with NaHCO3 solution. Effervescence with NaHCO3 indicates the presence of a carboxylic acid. The product is a ketone, not a carboxylic acid. So, (A) is incorrect.
(B) (X) decolourises Br2 - water. Decolourisation of bromine water indicates the presence of a carbon-carbon double bond. The product is a saturated bicyclic ketone. It does not have a double bond. So, (B) is incorrect.
(C) Formation of major product involves nucleophilic attack. Yes, the formation of the major product involves the nucleophilic attack of the enolate carbon (C5) on the carbon bearing the leaving group (C6). So, (C) is correct.
(D) The given reaction involves nucleophilic substitution. The reaction involves intramolecular nucleophilic attack leading to cyclization and formation of a new ring. This can be considered as an intramolecular nucleophilic substitution reaction where the enolate acts as a nucleophile and OTs is the leaving group.
