Question
Question: Consider the given reaction, the product ‘A’ is: 
A. C6H5OH
B. C6H5COCH3
C. C6H5Cl
D. C6H5CHO
Solution
. Benzoyl chloride can also be known as benzene carbonyl chloride, it is an organochlorine compound with the formula C6H5COCl. It is a colorless, fuming liquid with an irritating odor.
Complete step by step answer:
Benzoyl chloride is produced from benzotrichloride using either water or benzoic acid. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.
C6H5CCl3+H2O→C6H5COCl+2HCl
C6H5CCl3+C6H5COOH→2C6H5COCl+2HCl
Reaction given in the question is example of Rosenmund reduction
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. This reaction is generally a hydrogenolysis which is catalysed by palladium on barium sulfate which can be called the Rosenmund catalyst. Barium sulfate has a low surface area which reduces the activity of the palladium and prevents over-reduction. However, for certain reactive acyl chlorides the activity must be reduced further, by the addition of a poison. Originally this was thioquinanthrene although thiourea has also been used. Deactivation is required because the system must reduce the acyl chloride but not the subsequent aldehyde. If further reduction does take place it will create a primary alcohol which would then react with the remaining acyl chloride to form an ester. Rosenmund catalyst can be prepared by reduction of palladium (II) chloride solution in the presence of BaSO4.
Thus the product A in this reaction is
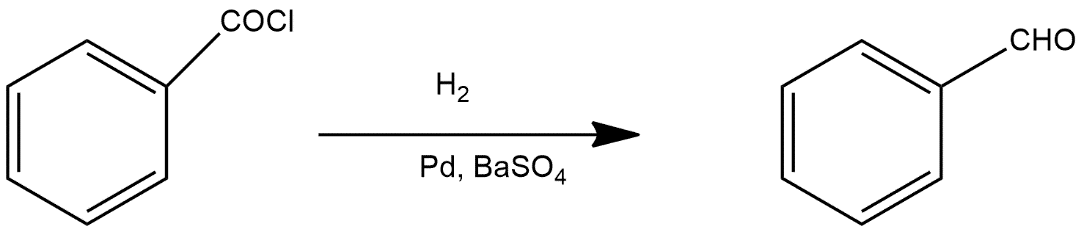
So, the correct answer is “Option D”.
Note: It reacts with water to produce hydrochloric acid and benzoic acid. Benzoyl chloride is a typical acyl chloride. It reacts with alcohols to give the corresponding esters. Similarly, it reacts with amines to give the amide.
