Question
Question: Consider the following two complex ions: \[{\left[ {Co{F_6}} \right]^{3 - }}\] and \[{\left[ {Co{{\l...
Consider the following two complex ions: [CoF6]3− and [Co(C2O4)3]3−. Which of the following statement(s) is/are false?
I. Both are octahedral.
II. [Co(C2O4)3]3− is diamagnetic while [CoF6]3− is paramagnetic.
III. Both are outer orbital complexes.
IV. In both the complexes, the central metal is in the same oxidation state.
A. Both (II) and (III)
B. I), (III) and (IV)
C. Only (III) [Comment: the option is wrong, it should be only (II)]
D. Both (III) and (IV)
E. (I), (II) and (IV)
Solution
These are coordination compounds of cobalt (Co). The first complex is called hexafluoro cobalt and the second is the tris oxalato cobalt complex. Both of them are anionic complexes.
Complete step by step answer: In both the complexes cobalt is the central metal ion. In order to understand the characteristics of the given complexes we have to identify the type of coordination between the ligand and the central metal ion. In [CoF6]3− the ligand is the fluoride ion and in [Co(C2O4)3]3− the ligand is the oxalate ion. Let us evaluate the statements one by one.
-Both are octahedral.
In [CoF6]3− the ligand fluoride is a monodentate ligand and can form only one coordinate bond. Thus the coordination number of the complex is , C.No = 6×1=6.
In [Co(C2O4)3]3− the ligand oxalate is a bidentate ligand and can form two coordinate bonds. Thus the coordination number of the complex is, C.No. =3×2=6.
Hence both the complexes are octahedral.
- [Co(C2O4)3]3− is diamagnetic while [CoF6]3− is paramagnetic.
In both the complexes the central metal ion is in +3 oxidation state. The electronic configuration of cobalt and Co3+ are:
Co=[Ar]3d74s2
Co3+=[Ar]3d6
In Co3+ the two electrons are lost from 4s orbital and one electron from 3d orbital.
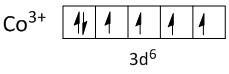
Both ligands, fluoride and oxalate are weak field ligands and cannot pair up the electrons of the central metal ion. Thus the electrons in the shell of 3d orbitals remain unpaired. So both complexes are paramagnetic.
-Both are outer orbital complexes.
As no pairing of electrons takes in the inner 3d orbital of Co3+, the electrons from ligands will fill up the outer orbitals of4s, 4p and4d. This makes the orbitals of Co3+ sp3d2 hybridized.
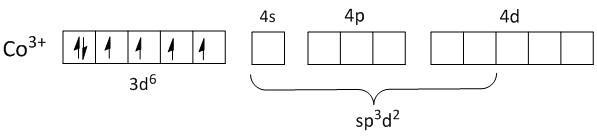
Thus both the complexes are outer orbital complexes.
IV. In both the complexes, the central metal is in the same oxidation state.
In [CoF6]3− the oxidation state of Co is:
x+6(−1)=−3 , (fluoride caries −1 charge)
x=+3 , the oxidation state is +3.
In [Co(C2O4)3]3−the oxidation state of Co is:
x+3(−2)=−3
x=+3 , the oxidation state is+3. Thus in both complexes the central metal has +3 oxidation state.
Hence, the option C is the correct answer, the false statement is only (II) as both complexes are paramagnetic.
Note: The number of ligands which combine with the central metal ion is referred as the coordination number of the complex. From every ligand the metal ion accepts electrons and forms coordinate covalent bonds. The ligands are Lewis bases and are electron donors.
