Question
Question: Consider the following statements in respect of \({[CoC{l_6}]^{4 - }}\) complex ion- I: It is para...
Consider the following statements in respect of [CoCl6]4− complex ion-
I: It is paramagnetic
II: It is low spin-complex.
III: Oxidation number of cobalt is -4
IV: The coordination number of cobalt is 6.
Select correct statements.
A. All of the above
B. III and IV only
C. I and IV only
D. I and II only
Solution
Firstly we will find the oxidation number of the cobalt in the given complex compound and then we will make electronic configuration of the cobalt ion. After this we will check for the low spin and high spin of the complex by knowing the type of ligand. And finally we will arrange the electron to find out the nature of the compound viz. paramagnetic or diamagnetic.
Complete answer:
As we all know that this is a given complex compound. Now we will find the oxidation number of the cobalt in the given compound.
Let oxidation number of cobalt will be x
And we all know that oxidation number of the chlorine ion is -1
So , to balance the compound positive and negative charge should be equal,
Hence,
x−6=−4 x=2
We have found the oxidation number of cobalt is +2 in the given compound.
Now we will find the electronic configuration of Co+2ion.
Co+2⇒1s2,2s2,2p6,3s2,3p6,4s2,3d5
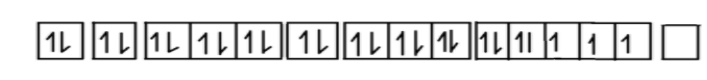
∴ We all know that chlorine is a weak field ligand so pairing of electrons will not occur in this complex compound.
So, there will be 3 lone pair of electrons,
Hence the given compound will have paramagnetic character.
∴ We have discussed that the given compound has weak field ligands. And weak field ligands always form the high spin complex or outer orbital complex.
So, the given compound will have a high spin character.
We have previously found that the oxidation number of cobalt will be +2
∴ Cobalt is attracted to the six chlorine ions in the given complex. hence, coordination number of cobalt will be 6.
Option number C will be the correct answer because it satisfies all the answers we have discussed.
Note:
Paramagnetic character: If in the electronic configuration of a compound we will get the lone pair(single) of electron then that compound will show the paramagnetic behavior and will be attracted towards the magnet.
Coordination number is defined as the number of combining atoms to the cations.
Oxidation number is defined as the number of electrons lost by the cation.
