Question
Question: Consider the following set of reactions \(C{H_3}COOH\xrightarrow{{SOC{l_2}}}A\xrightarrow[{Benzene...
Consider the following set of reactions
CH3COOHSOCl2AAnhyd.AlCl3BenzeneBHCNCHOHD
The structure D will be
A.)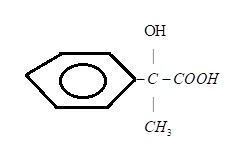
B.)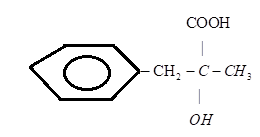
C.)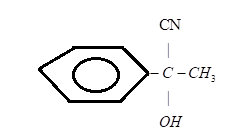
D.)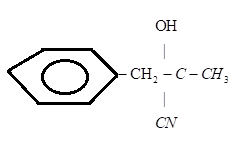
Solution
When thionyl chloride that is SOCl2 reacts with a carboxylic acid then the −OH of the carboxylic acid replaces with the chlorine to form an acid chloride or acyl chloride in the product. The benzene with AlCl3 reacts with this acyl chloride then chlorine is replaced by the benzene group.
Complete answer:
In the given set of reactions, the first reactant is a carboxylic acid. The SOCl2 has the chemical name as Thionyl Chloride. When thionyl chloride (SOCl2) reacts with carboxylic acid then the product formed is acid chloride or we can say it is acyl chloride with general chemical formulas as R−OCl. In this reaction, −OH of the carboxylic is replaced by the −Cl . Hence, this reaction can be written as:
CH3COOHSOCl2CH3COCl
When the acyl chloride that is formed in the above reaction reacts with the benzene in the presence of aluminium trichloride (AlCl3) then the benzene ring will attach in place of chlorine. This reaction is known as Friedel craft acylation. In Friedel crafts acylation, when acyl group that is CH3COCl reacts with an aromatic compound then the chlorine of the acyl chloride gets replaced with the aromatic compound that is benzene here. So, the reaction can be shown as:
CH3COClBenzeneAnhyd.AlCl3C6H5COCH3
When C6H5COCH3 reacts with HCN then the CN combines with the carbon of carboxylic group and H combines with oxygen of the carboxylic group to form C6H5C(OH)(CH3)CN. This reaction can be represented as:
C6H5COCH3HCNC6H5C(OH)(CH3)CN
When the formed product that is C6H5C(OH)(CH3)CN reacts with water then −CN will be replaced by the −COOH group. This reaction can be represented as:
C6H5C(OH)(CH3)CNHOHC6H5C(OH)(CH3)COOH
Therefore, the set of reactions can be written as:
CH3COOHSOCl2CH3COClAnhyd.AlCl3BenzeneC6H5COCH3 HCNC6H5C(OH)(CH3)CNHOHC6H5C(OH)(CH3)COOH
Therefore, the product D is C6H5C(OH)(CH3)COOH
Hence, the option A.) is the correct answer.
Note: Always remember that when any acyl chloride that is of general formula as R−OCl reacts in the presence of AlCl3 with an aromatic compound then it is Friedel craft acylation and when an alkyl halide reacts with these reagents the it is called as Friedel craft alkylation.
