Question
Question: Consider the following sequence of reactions and find A and B. 
Solution
Think about how when a ketone reacts with a hydroxylamine, it is converted into an oxime. This oxime then reacts with an acid to give an amide functional group inside the compound. Try to predict the products based on this.
Complete answer:
On the side of the reactants, have one molecule of cyclohexanone along with a standard hydroxylamine molecule. These two molecules combine on heating to form an oxime molecule through an intermediate compound that has both, a hydroxyl group as well as a hydroxylamine group attached to the carbonyl carbon. Let us look at the reaction mechanism in detail.
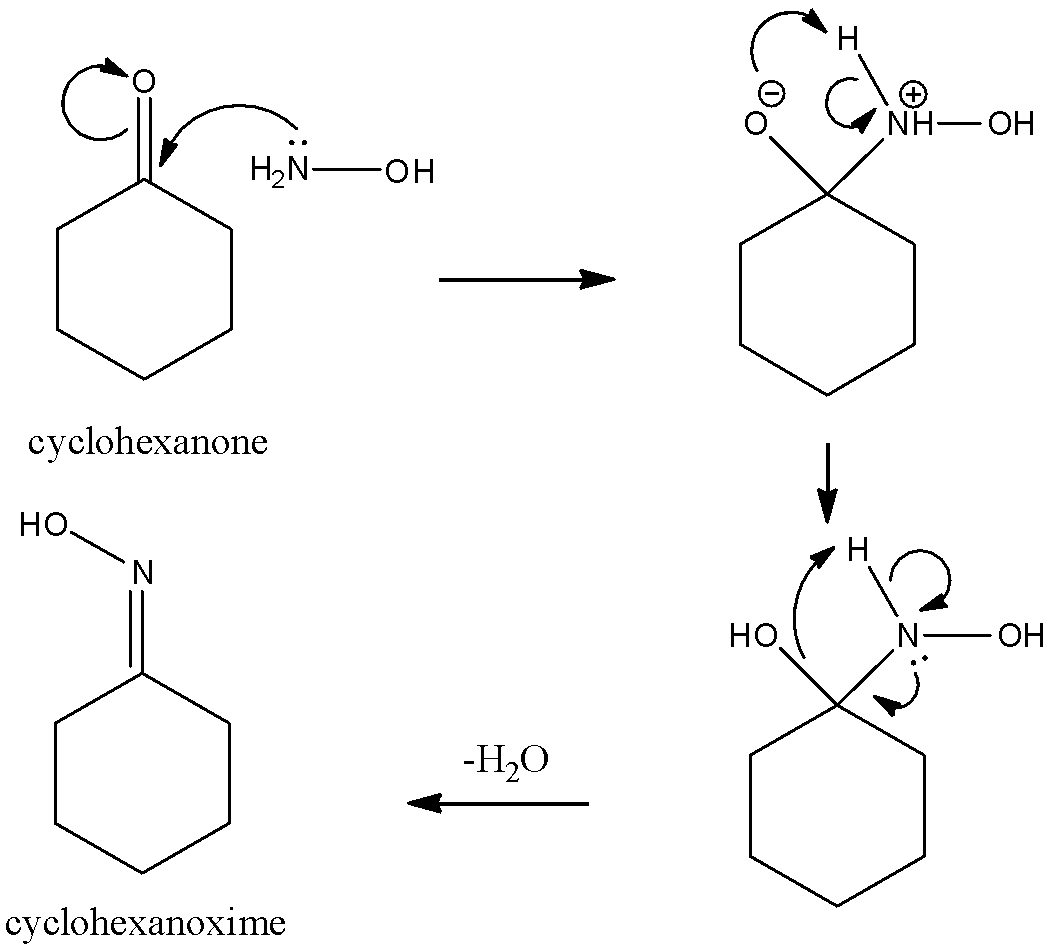
Here, we can see that the lone pair on the nitrogen atom in the hydroxylamine initially attacks the carbonyl carbon of the ketone and then after a bit of intramolecular shifting of electrons and the loss of a water molecule, we get an oxime. So the first product A is an oxime.
This oxime then reacts with an acid, which gives us a lot of hydronium ions, these hydronium ions then react with this oxime to give an amide. The amide that will be formed in this case will be caprolactam. Let us look at the reaction mechanism for this process.
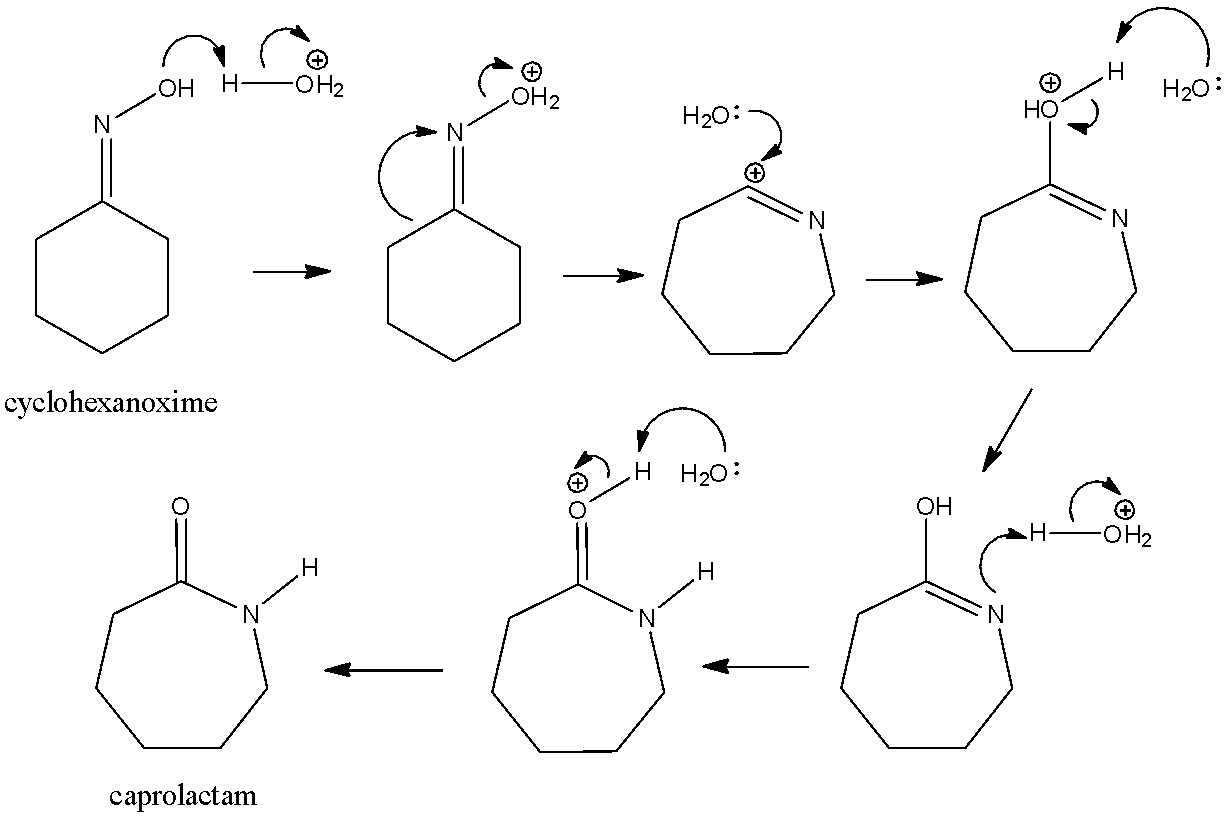
Here, we can see that the cyclohexanone reacts with the hydronium ions as well as the water molecules and forms multiple intermediates. After the reaction is complete, we get a final amide that is known as caprolactam.
Hence, the answer to this question is that A is cyclohexanone and B is caprolactam.
Note:
In the second part of the reaction, we are always considering hydronium (H3O+) ions since when any acid is diluted in water, it ionizes and forms hydronium ions which then reacts with the reactant molecules. Hence, instead of considering the reaction with sulfuric acid, we will consider it with hydronium ions.
