Question
Question: Consider the following scheme involving oxides and oxy-acids of nitrogen. Out of the following which...
Consider the following scheme involving oxides and oxy-acids of nitrogen. Out of the following which reactions are disproportionately?

A. 2, 10
B. 2,3
C. 1,11
D. 13,14
Solution
An oxide is a chemical compound which contains at least one oxygen atom with any another element. Oxide is a di anion of oxygen represented as in this state the oxygen is present in -2 oxidation state and acts as an anion which carries negative charge.
Complete answer:
Disproportionation is defined as the type of redox reaction in which species are simultaneously reduced and oxidized to form two different products. Redox reaction can be defined as that chemical reaction in which electrons are transferred between the reactants participating in the chemical reaction. The transfer of electrons involves the changes in the oxidation state of the reacting species.
The answer should be calculated by the concept of oxidation state which can be defined as the loss of electrons or we can say that increase in the oxidation state of an atom, ion or molecule while reduction can be defined as the gain of electrons or decreases in the oxidation state of any given atom, ion or molecule.
Here the oxidation state of nitrogen in different type of compounds can be calculated by:
N2= 0
NH3= -3
NO= +2
HNO2= +3
NO2,N2O4= +4
N2O5= +5
HNO3= +5
This will lead to the following graph:
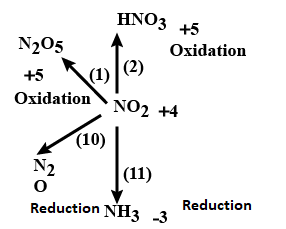
This shows that option A and option C are the correct answer.
Note:
Oxides can be formed due to the electronegative nature of oxygen which forms stable chemical bonds with almost all metals and they give corresponding oxides of that metal. Ratio of metal oxide tells us how many metals and oxygen atoms are connected with each other.
