Question
Question: Consider the following reactions: $A_x + yB_2 \xrightarrow[supply \ of \ air]{limited} compound \ '...
Consider the following reactions:
Ax+yB2limitedsupply of aircompound ′P′+zB2excess airCompound ′Q′
If atomic number of elements A and B are 15 and 8 respectively, then according to the given information the correct statements(s) is / are:
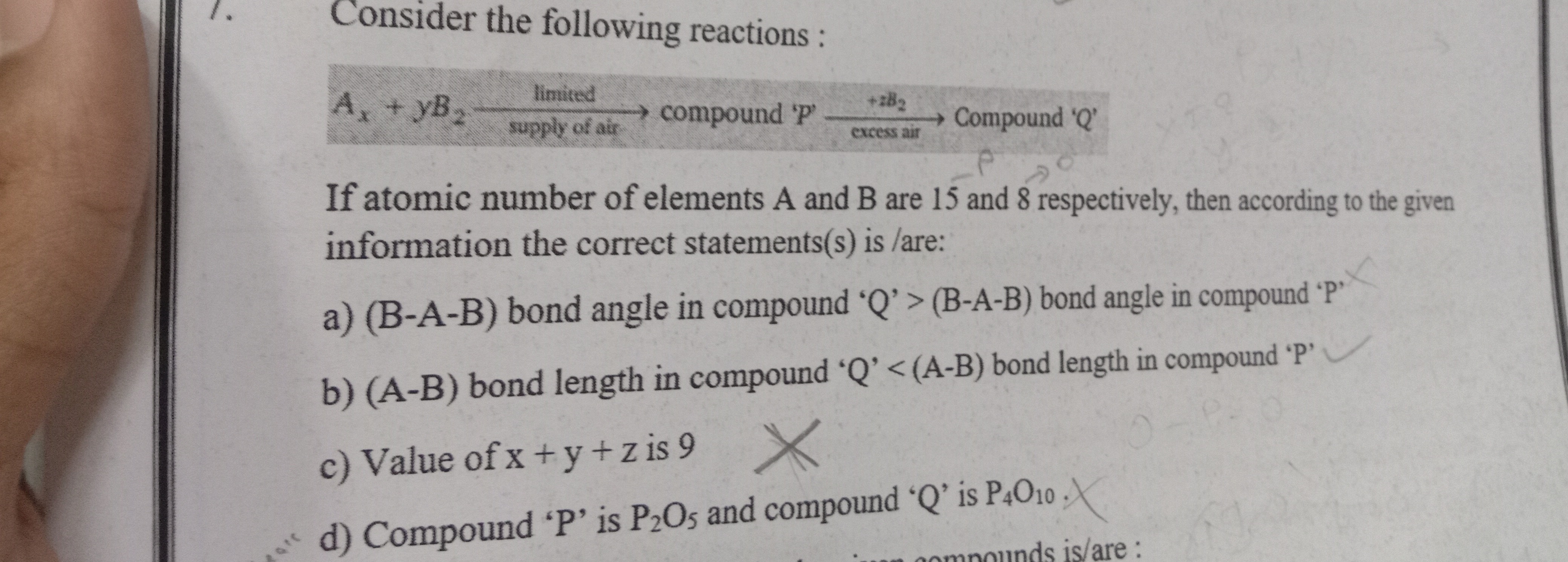
(B-A-B) bond angle in compound 'Q' > (B-A-B) bond angle in compound 'P'
(A-B) bond length in compound 'Q' < (A-B) bond length in compound 'P'
Value of x + y + z is 9
Compound 'P' is P2O5 and compound 'Q' is P4O10
b
Solution
Elements A and B are identified as Phosphorus (P) and Oxygen (O) respectively based on their atomic numbers. The reactions show that phosphorus reacts with limited oxygen to form P4O6 (compound 'P') and with excess oxygen to form P4O10 (compound 'Q'). The values of x, y, and z are determined from the balanced equations as x=4, y=6, and z=2. Statement (c) is incorrect as x+y+z=12=9. Statement (d) is incorrect because 'P' is P4O6, not P2O5. Statement (a) is incorrect as the O-P-O bond angles in P4O10 are not consistently greater than those in P4O6. Statement (b) is correct because P4O10 contains shorter P=O double bonds (approx. 143 pm) compared to the P-O single bonds in P4O6 (approx. 165 pm).
