Question
Question: Consider the following reactions: a.\({(C{H_3})_3}CCH(OH)C{H_3}\xrightarrow{{conc.{H_2}S{O_4}}}\) ...
Consider the following reactions:
a.(CH3)3CCH(OH)CH3conc.H2SO4
b.(CH3)2CHCH(Br)CH3alc.KOH
c.(CH3)2CHCH(Br)CH3(CH3)3COΘK⊕
d.(CH3)2C(OH)−CH2−CHO△
Which of the above reactions will not produce Saytzeff product?
A.(c) only
B.(b) and (d)
C.(d) only
D.(a), (c) and (d)
Solution
We have to know that a Saytzeff product is basically an alkene compound which has undergone many substitution reactions to form the alkene product. From Saytzeff rule, a stable alkene is formed when removal of hydrogen from beta carbon has a low number of hydrogen atoms. For all the given reactions in the question, first will proceed the reaction and check whether the reactants produce alkenes or not.
Complete answer:
Let’s process the first reaction:
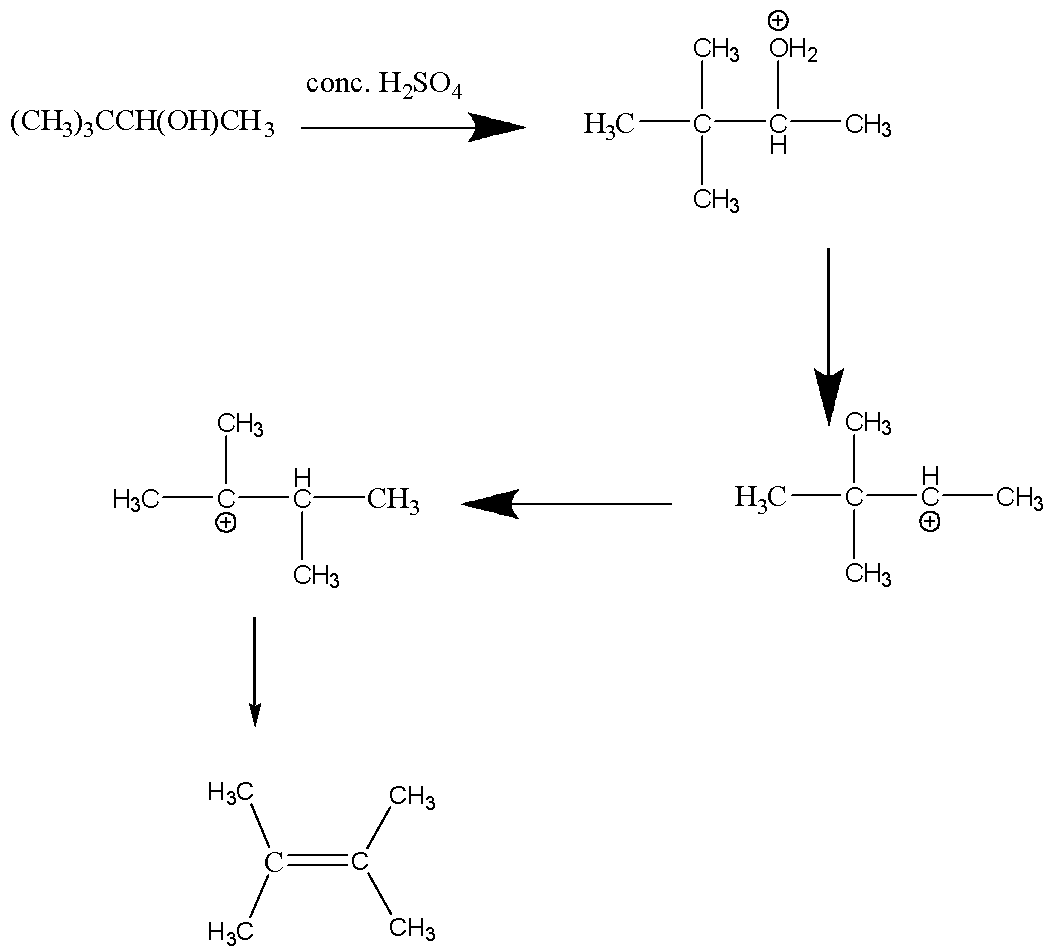
In the above reaction, a Saytzeff product is formed.
Let’s see the second reaction,
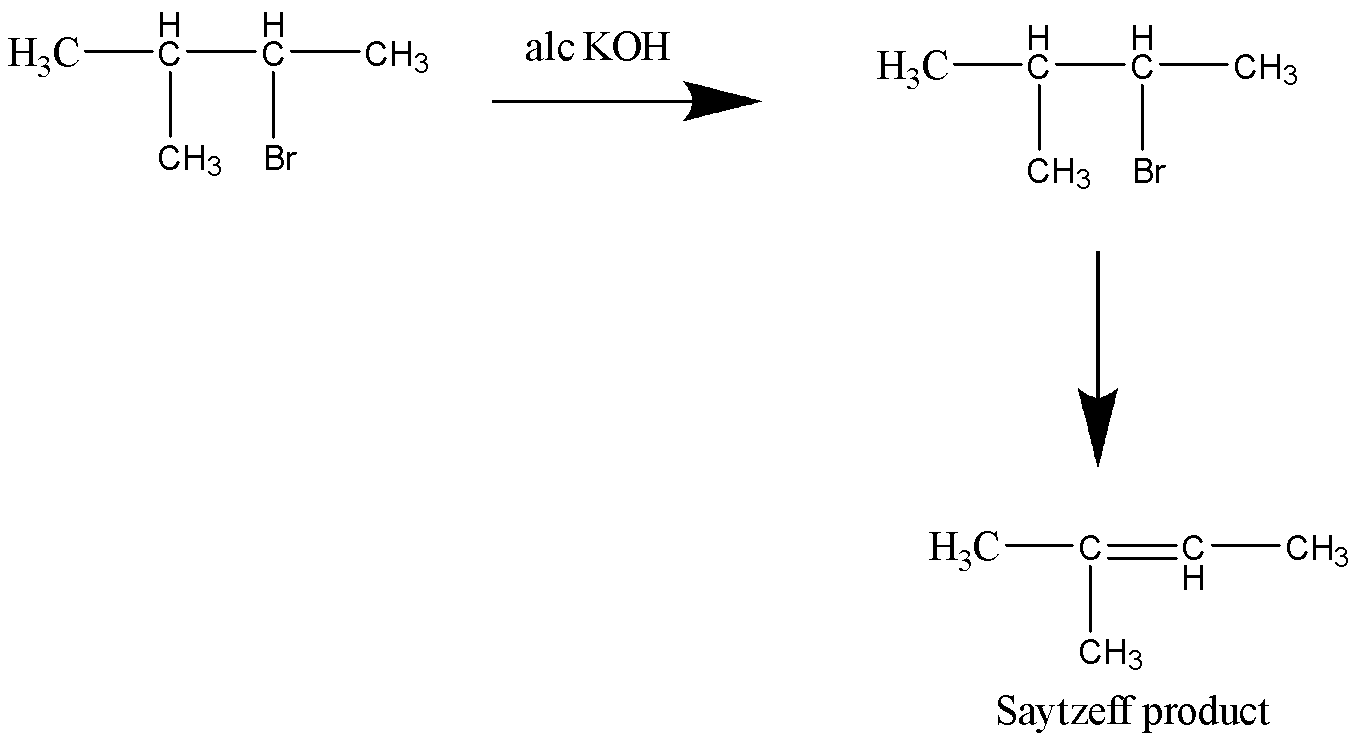
This reaction too yields a Saytzeff product.
Let’s consider the third reaction,
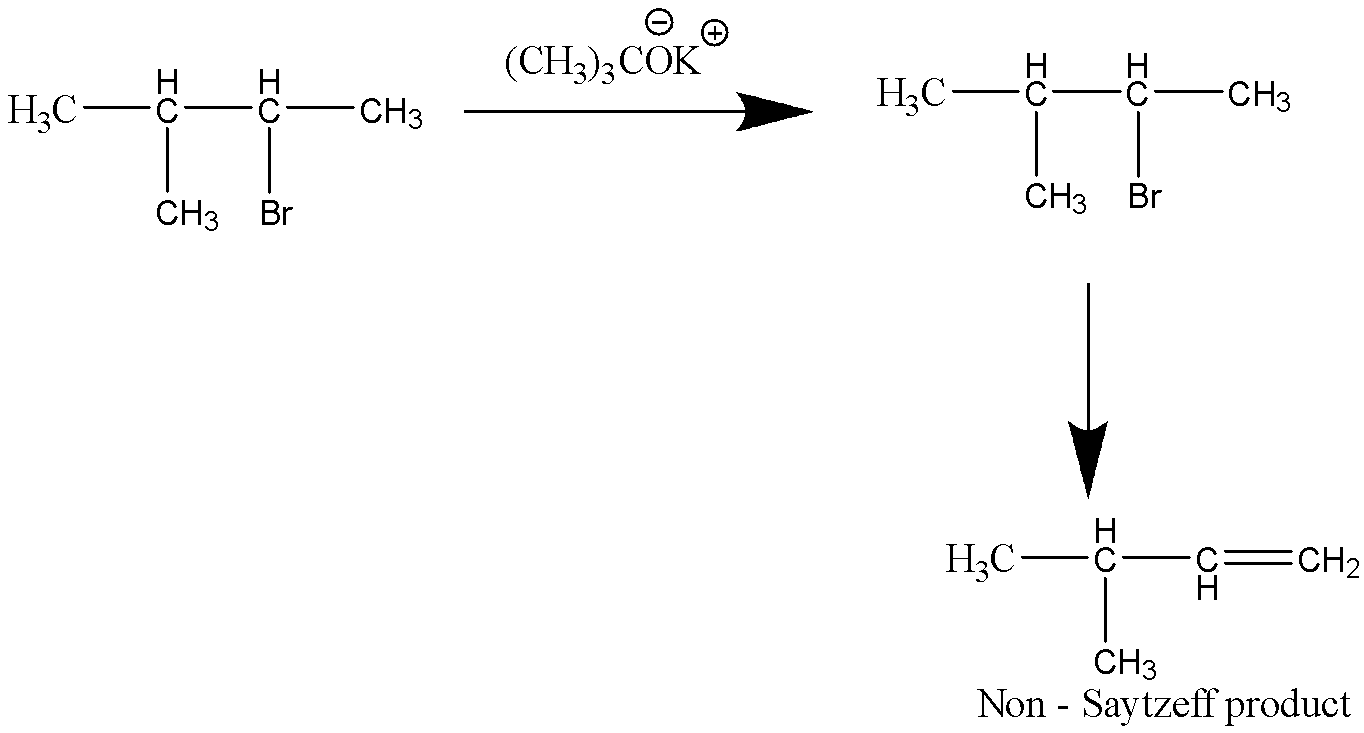
This reaction does produce an alkene compound, but not in a tertiary carbon atom. Hence, this reaction does not produce Saytzeff product.
Let’s consider the last reaction as well,
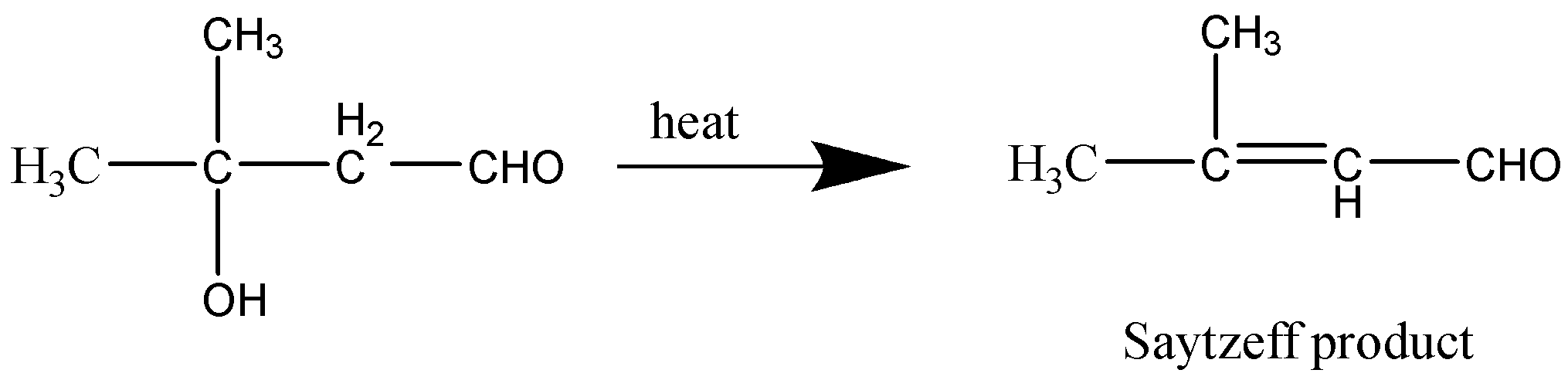
This reaction too produces a Saytzeff product.
Hence, the correct answer to the question is (A) – reaction (c) only. Rest all reactions yields Saytzeff product.
Note:
We must remember that the Saytzeff rules states that more substituted alkene will be the major product. Higher the degree of substitution of alkene will form the major product and the rest the minor product. The reaction number (c) does not yield saytzeff product, because the double bonding is in secondary position, while the carbon atom at tertiary position is more reactive and less stable. During elimination reactions, generally Saytzeff rule is considered.
