Question
Question: Consider the following reaction: $xMnO_4^- + yI^- + zH^+ \longrightarrow pMn^{2+} + qIO_3^- + rH_2O...
Consider the following reaction:
xMnO4−+yI−+zH+⟶pMn2++qIO3−+rH2O
The correct ratio x:y in the balanced equation is
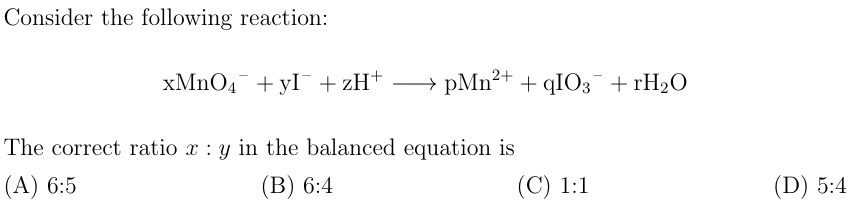
6:5
6:4
1:1
5:4
6:5
Solution
The given reaction is:
xMnO4−+yI−+zH+⟶pMn2++qIO3−+rH2O
We will balance this redox reaction using the ion-electron method in acidic medium.
Step 1: Identify oxidation and reduction half-reactions.
- In MnO4−, Mn is in the +7 oxidation state. In Mn2+, Mn is in the +2 oxidation state. Thus, MnO4− is reduced.
- In I−, I is in the -1 oxidation state. In IO3−, let the oxidation state of I be 'a'. Since oxygen is -2, we have a + 3(-2) = -1, which gives a - 6 = -1, so a = +5. Thus, I− is oxidized.
Reduction half-reaction: MnO4−⟶Mn2+ Oxidation half-reaction: I−⟶IO3−
Step 2: Balance atoms other than O and H.
- Manganese atoms are balanced.
- Iodine atoms are balanced.
Step 3: Balance oxygen atoms by adding H2O.
- Reduction: MnO4−⟶Mn2++4H2O (4 oxygen atoms on the left, so add 4 H2O to the right)
- Oxidation: I−+3H2O⟶IO3− (3 oxygen atoms on the right, so add 3 H2O to the left)
Step 4: Balance hydrogen atoms by adding H+ (since it's an acidic medium).
- Reduction: MnO4−+8H+⟶Mn2++4H2O (8 hydrogen atoms on the right from 4H2O, so add 8 H+ to the left)
- Oxidation: I−+3H2O⟶IO3−+6H+ (6 hydrogen atoms on the left from 3H2O, so add 6 H+ to the right)
Step 5: Balance charge by adding electrons (e−).
- Reduction: MnO4−+8H+⟶Mn2++4H2O
- Charge on left: (-1) + (+8) = +7
- Charge on right: +2
- To balance, add 5 electrons to the left side: MnO4−+8H++5e−⟶Mn2++4H2O
- Oxidation: I−+3H2O⟶IO3−+6H+
- Charge on left: -1
- Charge on right: (-1) + (+6) = +5
- To balance, add 6 electrons to the right side: I−+3H2O⟶IO3−+6H++6e−
Step 6: Equalize the number of electrons transferred in both half-reactions.
The least common multiple of 5 and 6 is 30.
- Multiply the reduction half-reaction by 6: 6(MnO4−+8H++5e−⟶Mn2++4H2O) 6MnO4−+48H++30e−⟶6Mn2++24H2O
- Multiply the oxidation half-reaction by 5: 5(I−+3H2O⟶IO3−+6H++6e−) 5I−+15H2O⟶5IO3−+30H++30e−
Step 7: Add the balanced half-reactions and cancel common species.
(6MnO4−+48H++30e−⟶6Mn2++24H2O) + (5I−+15H2O⟶5IO3−+30H++30e−)
6MnO4−+48H++30e−+5I−+15H2O⟶6Mn2++24H2O+5IO3−+30H++30e−
Cancel 30e− from both sides. Cancel 30H+ from 48H+ on the left, leaving 18H+ on the left. Cancel 15H2O from 24H2O on the right, leaving 9H2O on the right.
The balanced equation is:
6MnO4−+5I−+18H+⟶6Mn2++5IO3−+9H2O
Step 8: Determine the ratio x:y.
From the balanced equation, x=6 and y=5. The ratio x:y is 6:5.
