Question
Question: Consider the following reaction sequence, isomers are: 
(A) C and E
(B) C and D
(C) D and E
(D) C, D and E
Solution
The given acid is succinic acid. To solve this we must know the role of all the reagents on succinic acid. After we find all the products we have to find the compounds that are isomers of each other. The compounds having the same formula but have different arrangements of atoms in their molecules are known as isomers.
Complete step by step solution:
We are given succinic acid. Succinic acid on reaction with phosphorus in presence of bromine gives a HVZ (Hell Volhard Zelinsky) product. During the reaction, the succinic acid is brominated at the alpha carbon. The reaction is as follows:
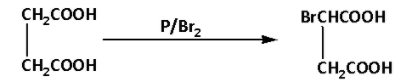
The product of the reaction is 2-bromosuccinic acid. Thus, A is 2-bromosuccinic acid.
2-bromosuccinic acid further reacts with alcoholic potassium hydroxide. During the reaction, potassium bromide and water are obtained as by-products. And one hydrogen and one bromine atom are eliminated. The reaction is as follows:

The product of the reaction is fumaric acid. Thus, B is fumaric acid.
Fumaric acid reacts with ozone in presence of water. During the reaction hydrogen gas is eliminated. The reaction is as follows:
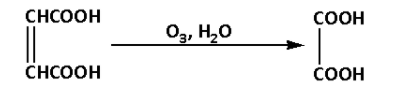
The product of the reaction is oxalic acid. Thus, C is oxalic acid.
Fumaric acid reacts with trifluoroperacetic acid. During the reaction, a racemic mixture of tartaric acid is produced. The reaction is as follows:
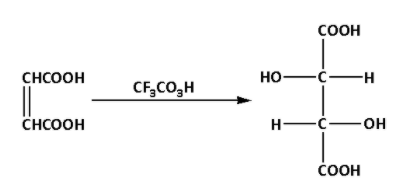
The product of the reaction is a racemic mixture of tartaric acid. Thus, D is a racemic mixture of tartaric acid.
Fumaric acid reacts with alkaline potassium permanganate. During the reaction, meso-tartaric acid is produced. The reaction is as follows:
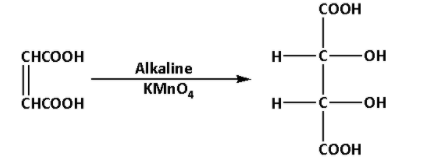
The product of the reaction is meso-tartaric acid. Thus, E is meso-tartaric acid.
The overall reaction sequence is as follows:
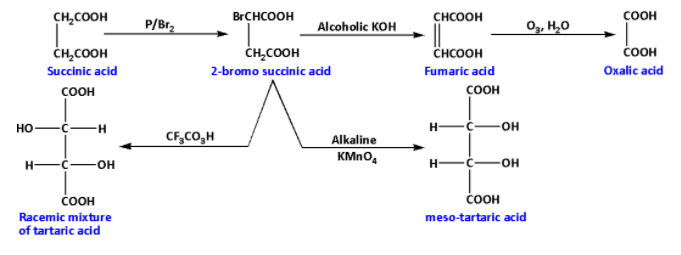
From the reaction sequence we can conclude that the racemic mixture of tartaric acid and meso-tartaric acid have the same formula but have different arrangements of atoms in their molecules. Thus, racemic mixtures of tartaric acid and meso-tartaric acid are isomers.
Thus, isomers are D and E.
Thus, the correct option is (C) D and E.
Note: The racemic mixture is an equal mixture of two mirror image isomers. The isomers are optically active in opposing directions. The racemic mixture of tartaric acid contains d-tartaric acid and l-tartaric acid.
