Question
Question: Consider the following reaction sequence: I. The compound 'P' is II. The compound 'P' on reaction ...
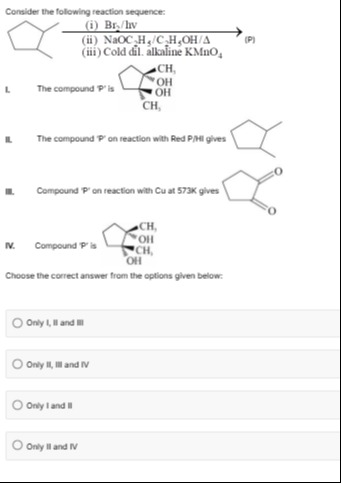
Consider the following reaction sequence:
I. The compound 'P' is
II. The compound 'P' on reaction with Red P/HI gives
III. Compound 'P' on reaction with Cu at 573K gives
IV. Compound 'P' is
Choose the correct answer from the options given below:
Only I and II
Only II and III
Only III and IV
Only II and IV
Only II and IV
Solution
The problem involves a reaction sequence starting from 1,2-dimethylcyclopentane and then analyzing the properties and reactions of the final product 'P'.
Step 1: Determine the structure of compound 'P'.
The starting material is 1,2-dimethylcyclopentane. The reaction sequence is: (i) Br₂/hν (Free radical bromination) (ii) NaOC₂H₅/C₂H₅OH/Δ (E2 elimination) (iii) Cold dil. alkaline KMnO₄ (Baeyer's reagent, syn-dihydroxylation)
-
Step (i): Br₂/hν
Free radical bromination occurs preferentially at the most substituted carbon (tertiary > secondary > primary). In 1,2-dimethylcyclopentane, the hydrogens on the carbons bearing the methyl groups (C1 and C2) are tertiary. There are two such tertiary hydrogens. Bromination will occur at one of these positions. Let's assume bromination at C1. The product is 1-bromo-1,2-dimethylcyclopentane.
-
Step (ii): NaOC₂H₅/C₂H₅OH/Δ
This is a strong base (sodium ethoxide) and heat, which promotes an E2 elimination reaction. The bromine atom is on C1. Beta-hydrogens are on C2 (one tertiary H) and C5 (two secondary H). According to Zaitsev's rule, the more substituted alkene is the major product. Removing a hydrogen from C2 leads to a double bond between C1 and C2, forming 1,2-dimethylcyclopent-1-ene. This is a tetrasubstituted alkene, which is the most stable and therefore the major product. The product is 1,2-dimethylcyclopent-1-ene.
-
Step (iii): Cold dil. alkaline KMnO₄ (Baeyer's reagent)
This reagent performs syn-dihydroxylation on an alkene, adding two hydroxyl groups to the same face of the double bond. For 1,2-dimethylcyclopent-1-ene, the double bond is between C1 and C2. Two -OH groups will be added to C1 and C2, respectively, in a syn fashion. Thus, compound 'P' is 1,2-dimethylcyclopentane-1,2-diol.
Step 2: Evaluate statements I, II, III, and IV.
-
Statement I: The compound 'P' is
The structure shown in statement I is 1,2-dimethylcyclopentane-1,2-diol, where the two methyl groups and the two hydroxyl groups are all shown in a specific stereochemical arrangement (one methyl up, one methyl down, both OHs up). Since Baeyer's reagent adds OH groups in a syn fashion, and the starting alkene is planar, the resulting diol will have the two OH groups on the same face. The relative stereochemistry of the methyl groups (cis or trans) in the starting 1,2-dimethylcyclopentane was not specified, and the bromination and elimination steps can lead to a mixture of isomers or specific isomers depending on conditions and stability. However, the question asks for the structure of 'P'. The structure shown in I is indeed 1,2-dimethylcyclopentane-1,2-diol. The representation with one CH3 up and one CH3 down indicates a trans relationship between the methyl groups, and both OHs are shown up. This is a plausible stereoisomer. Let's compare with IV. This shows the two methyl groups are trans to each other, and the two hydroxyl groups are cis to each other. This is consistent with syn-dihydroxylation of a trans-1,2-dimethylcyclopent-1-ene, but the alkene itself is 1,2-dimethylcyclopent-1-ene, where the methyl groups are on the double bond carbons. The representation in I shows the methyl groups on C1 and C2. One methyl is shown 'up' and the other 'down'. The two OH groups are shown 'up'. This structure represents 1,2-dimethylcyclopentane-1,2-diol.
-
Statement IV: Compound 'P' is
The structure shown in statement IV is also 1,2-dimethylcyclopentane-1,2-diol. This structure shows both methyl groups and both hydroxyl groups on the same face (e.g., all 'up'). This would result from syn-dihydroxylation of a cis-1,2-dimethylcyclopent-1-ene, which is not possible as the methyl groups are part of the double bond. However, if we interpret the structures as simply showing the connectivity and functional groups, both I and IV represent 1,2-dimethylcyclopentane-1,2-diol. Let's re-examine the image carefully. In statement I, the two methyl groups are shown trans to each other, and the two hydroxyl groups are shown cis to each other (both up). In statement IV, the two methyl groups are shown cis to each other, and the two hydroxyl groups are shown cis to each other (both up). Since the starting alkene is 1,2-dimethylcyclopent-1-ene, the two methyl groups are on the double bond carbons. After dihydroxylation, these carbons become chiral centers (if they are not already). The relative stereochemistry of the methyl groups in the product depends on the original stereochemistry of the 1,2-dimethylcyclopentane and the subsequent reactions. However, the product of syn-dihydroxylation of 1,2-dimethylcyclopent-1-ene will be 1,2-dimethylcyclopentane-1,2-diol. Let's assume the question is asking for a correct representation of P. The alkene 1,2-dimethylcyclopent-1-ene has the methyl groups attached to the double bond carbons. Upon syn-dihydroxylation, the two OH groups are added to C1 and C2 from the same face. The methyl groups are already attached to C1 and C2. If the methyl groups are in a fixed orientation relative to each other, then the diol will reflect that. But the alkene is planar. Let's consider the chirality. C1 and C2 become chiral centers. The structure in I (trans methyl, cis OH) and IV (cis methyl, cis OH) are two possible diastereomers of 1,2-dimethylcyclopentane-1,2-diol. Given the typical representation, the structure in IV (both methyls and both OHs on the same side) is more consistent with syn-addition. If the methyl groups are considered to be "up" from the plane of the ring (or "down"), then the OH groups would be added "up" (or "down") resulting in a cis-diol. Let's assume the question intends for IV to be the correct structure for P, as it shows both methyls and both OHs on the same side, which is a common way to depict the result of syn-addition. So, Statement IV is correct (assuming it depicts one of the possible stereoisomers correctly). This implies that statement I is incorrect as it represents a different diastereomer.
-
Statement II: The compound 'P' on reaction with Red P/HI gives
Red P/HI is a strong reducing agent that reduces alcohols (and many other functional groups) to alkanes. Compound 'P' is 1,2-dimethylcyclopentane-1,2-diol. Reduction with Red P/HI will remove the two hydroxyl groups and replace them with hydrogen atoms. The product will be 1,2-dimethylcyclopentane. The structure shown in statement II is 1,2-dimethylcyclopentane. So, Statement II is correct.
-
Statement III: Compound 'P' on reaction with Cu at 573K gives
Copper at 573K (300°C) is a reagent used for the dehydrogenation of alcohols. Primary alcohols give aldehydes. Secondary alcohols give ketones. Tertiary alcohols undergo dehydration to alkenes. Compound 'P' is 1,2-dimethylcyclopentane-1,2-diol. Both C1 and C2 are tertiary carbons (each bonded to two ring carbons, one methyl group, and one hydroxyl group). However, the question depicts the product as cyclopentane-1,2-dione. Let's re-examine the structure of P. C1 is bonded to a CH3 group, an OH group, and two ring carbons (C2 and C5). So C1 is a tertiary alcohol carbon. C2 is bonded to a CH3 group, an OH group, and two ring carbons (C1 and C3). So C2 is also a tertiary alcohol carbon. Tertiary alcohols, when heated with Cu at 573K, typically undergo dehydration to form alkenes, not dehydrogenation to form ketones. If it were a secondary alcohol, it would oxidize to a ketone. If it were a primary alcohol, it would oxidize to an aldehyde. The given product in statement III is cyclopentane-1,2-dione. This product (cyclopentane-1,2-dione) would be formed if the hydroxyl groups were on secondary carbons and the methyl groups were removed, or if the methyl groups were somehow oxidized or eliminated during the process. This is not consistent with the direct dehydrogenation of tertiary alcohols. Therefore, Statement III is incorrect.
Summary of Statements:
- I: Incorrect (likely due to stereochemistry or ambiguity, but IV is a better fit for the product from syn-addition).
- II: Correct (Reduction of diol to alkane).
- III: Incorrect (Tertiary alcohols dehydrate, not dehydrogenate to diones under these conditions).
- IV: Correct (Represents 1,2-dimethylcyclopentane-1,2-diol, consistent with syn-dihydroxylation).
Based on this analysis, statements II and IV are correct.
Final check on I vs IV: The structures are diastereomers. Both are 1,2-dimethylcyclopentane-1,2-diol. The question asks "The compound 'P' is". If there are multiple stereoisomers, any of them could be considered "the compound P" unless specific stereochemistry is implied. However, syn-dihydroxylation gives a cis-diol. In structure IV, both OH groups are 'up' and both CH3 groups are 'up'. This represents a cis-diol with cis-methyls, which is a possible result depending on how the methyl groups are positioned relative to the plane of the alkene. In structure I, the OH groups are 'up' (cis-diol), but the methyl groups are trans. This would imply syn-addition to a trans-1,2-dimethylcyclopent-1-ene, which is not possible for the methyl groups on the double bond. Therefore, IV is a more plausible representation of the product from syn-dihydroxylation.
Thus, II and IV are the correct statements.
