Question
Question: Consider the following reaction, \(Phenol\xrightarrow{Zn\text{ }dust}X\xrightarrow[anhyd.AlC{{l}_{...
Consider the following reaction,
PhenolZn dustXCH3Clanhyd.AlCl3Yalkaline KMnO4Z
The product Z is:
Solution
When phenol is made to react with zinc dust, it results in the elimination of the -OH group and the product X formed when treated with alkyl halide in the presence of anhydrous aluminum chloride undergoes alkylation and forms Y which when made to react with the alkaline potassium permanganate forms the product Z which is an acid. Now identify X, Y and Z.
Complete answer:
First of all, let’s discuss what is phenol. Phenols are the aromatic compounds consisting of the hydroxyl group i.e. the -OH group.
Now considering the statement;
When phenol is treated with the zinc dust it results in the formation of the benzene. The reaction occurs as;
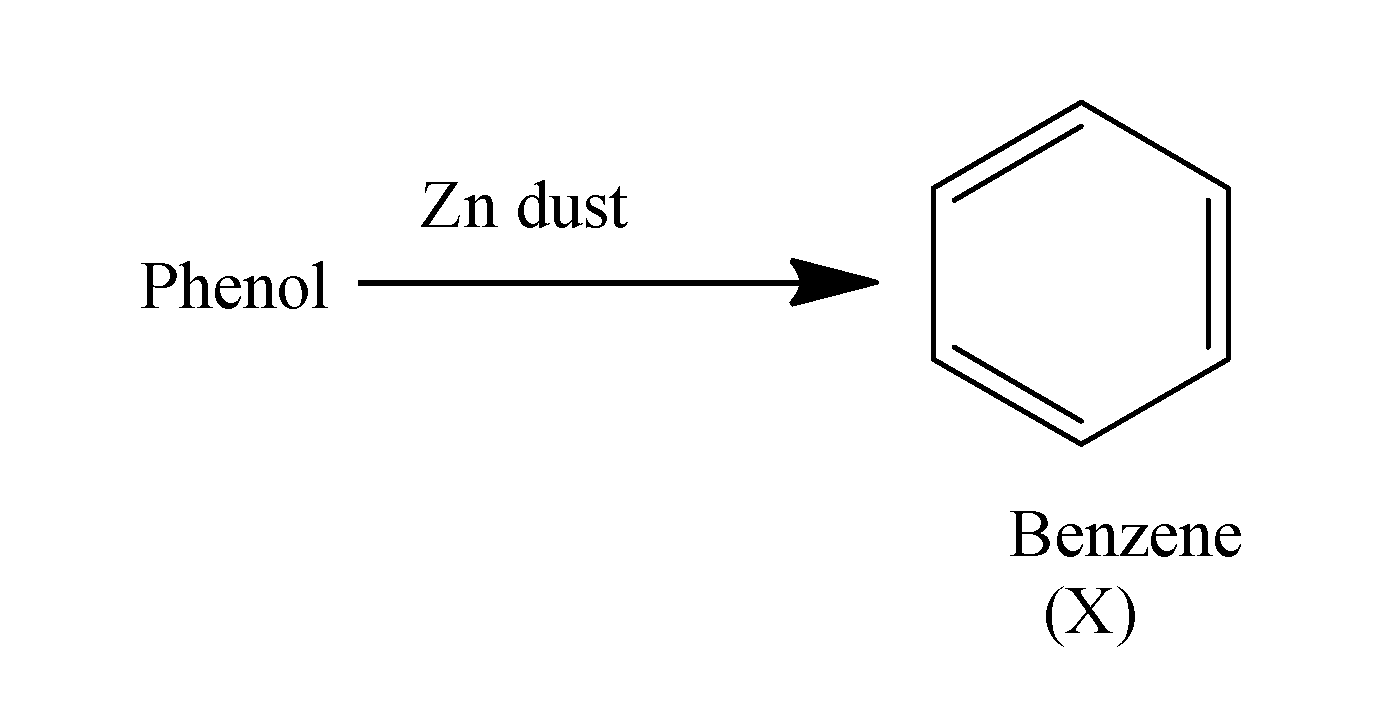
When this benzene ring is made to react with the methyl chloride in the presence of anhydrous aluminum chloride , it undergoes alkylation and results in the formation of methyl chloride i.e. toluene. The reaction occurs as;
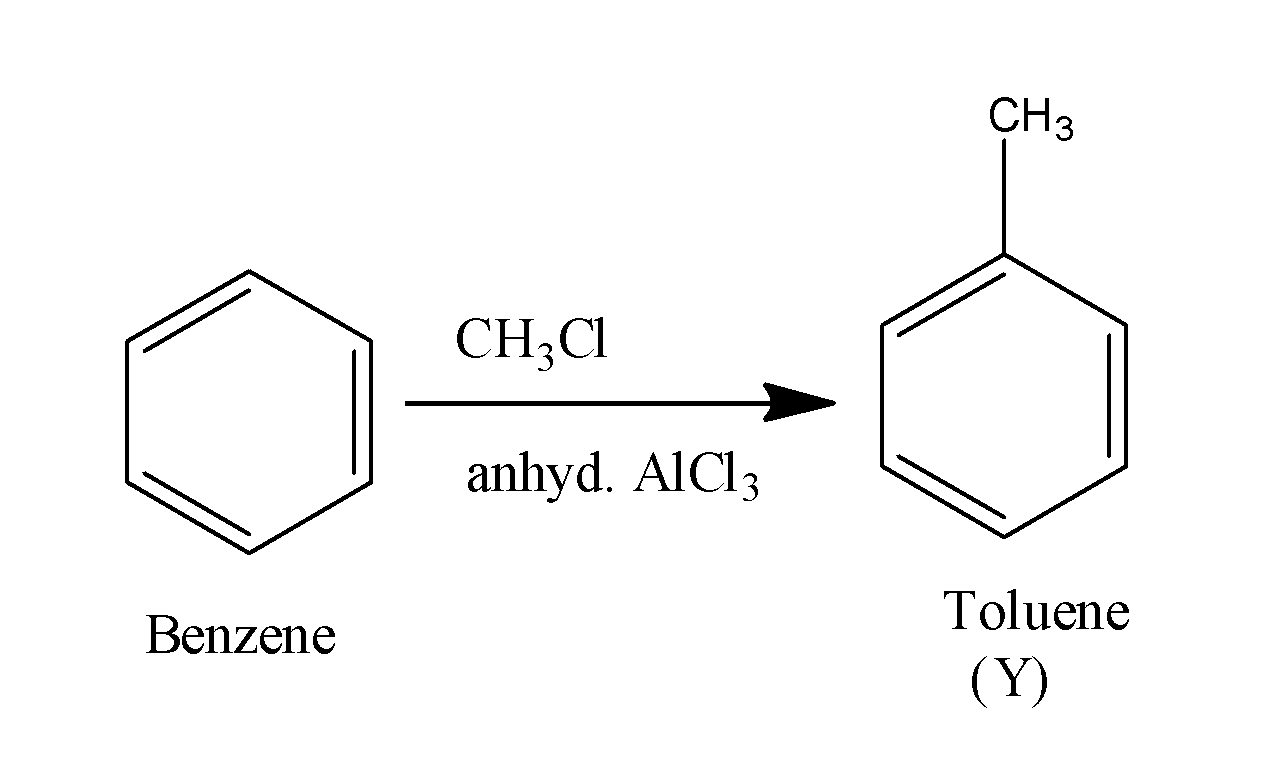
When this toluene is made to react with the alkaline potassium permanganate, it results in the formation of acid along with the removal of hydrogen gas. The reaction occurs as;
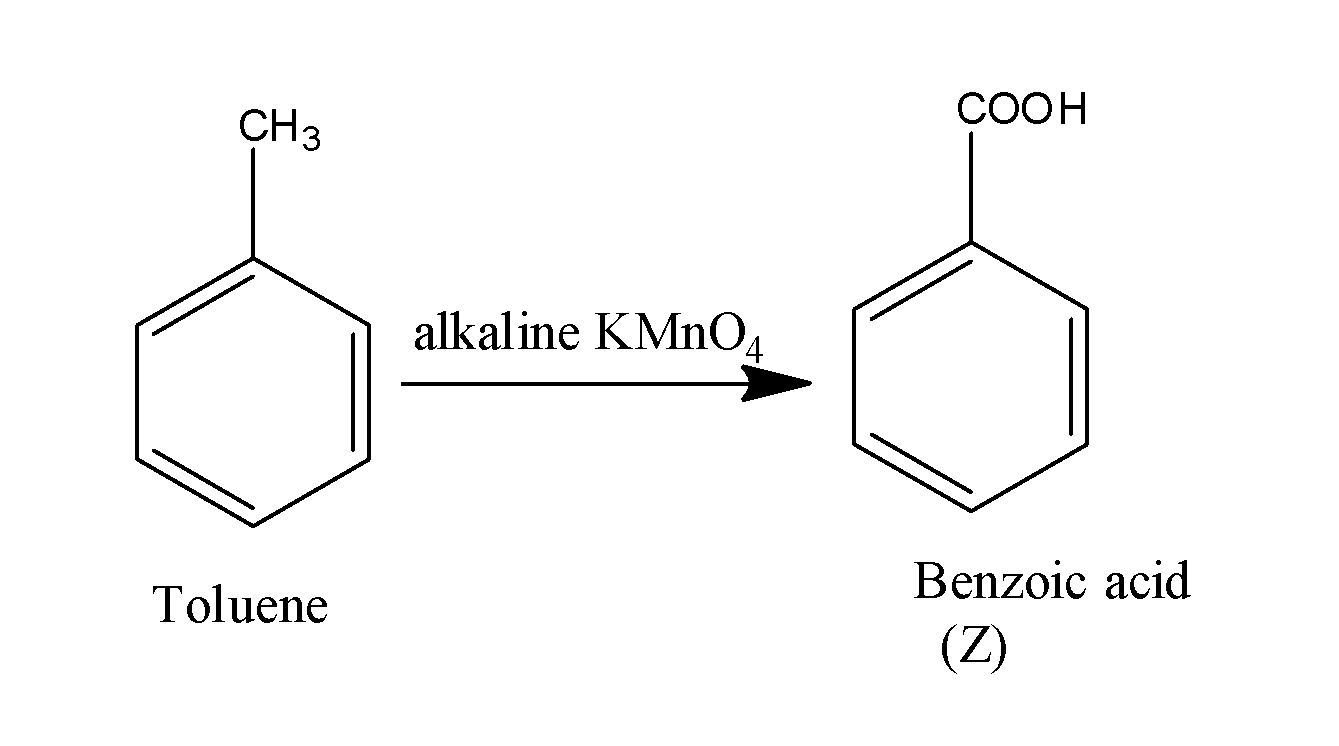
Thus, the overall reaction occurs as;
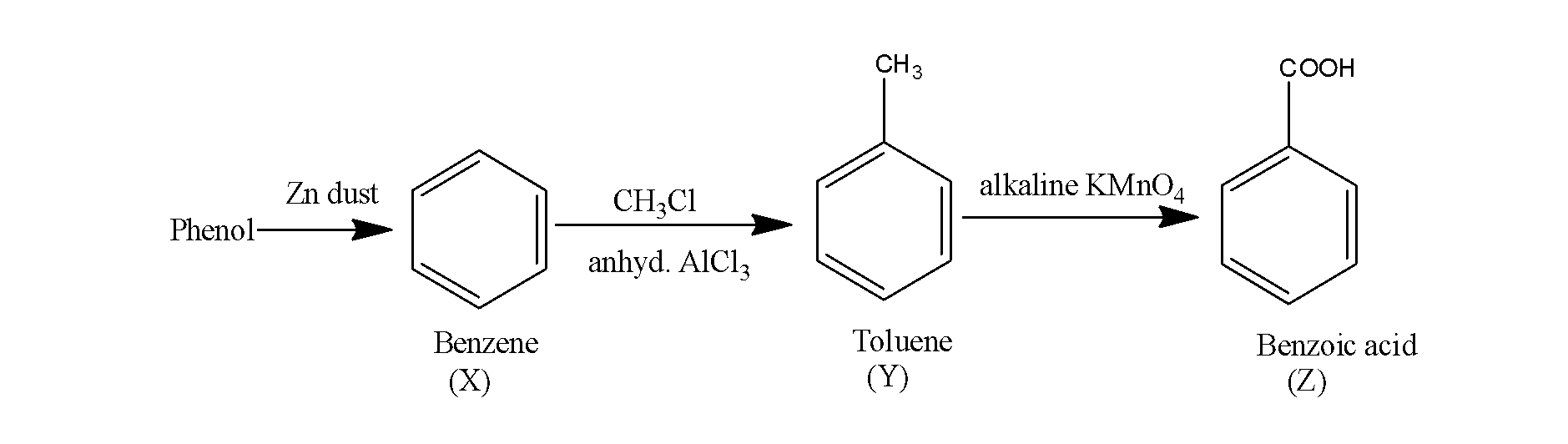
So, the product Z is benzoic acid.
Hence, option (b) is correct.
Note: The reaction of benzene with the alkyl chloride in the presence of aluminum chloride is known as the friedel craft alkylation and such reactions are known as the electrophilic substitutions reactions i.e. the hydrogen atom attached to the carbon atom of the benzene ring is replaced with the alkyl group.
