Question
Question: Consider the following reaction If total number of halogen atom in major product (A) is x and degre...
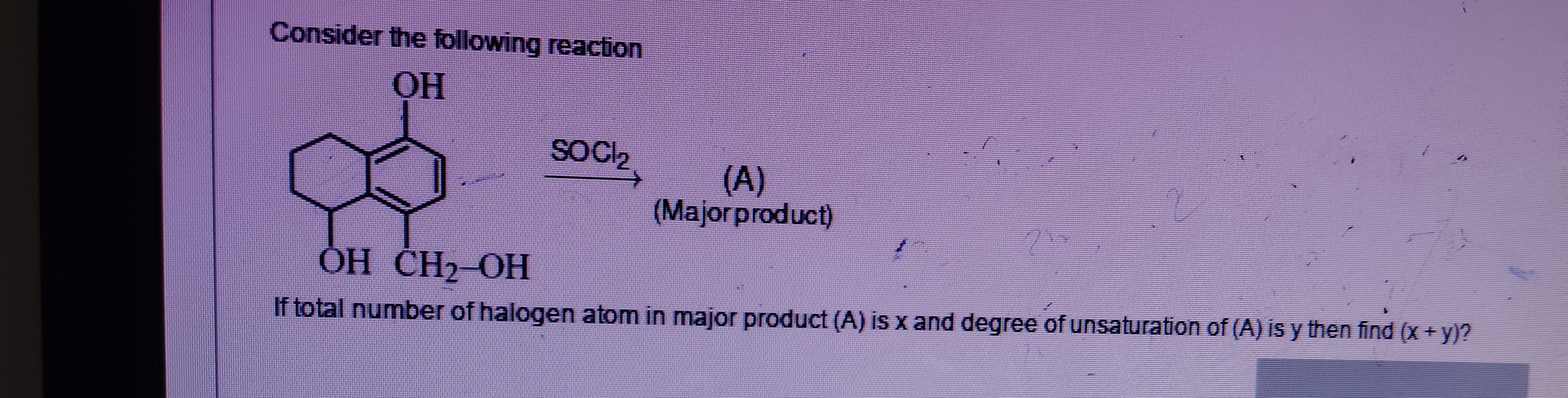
Consider the following reaction
If total number of halogen atom in major product (A) is x and degree of unsaturation of (A) is y then find (x + y)?
7
Solution
The given reaction involves the reaction of a compound with two hydroxyl groups and a −CH2OH group with SOCl2. The starting material is a derivative of 1,2,3,4-tetrahydronaphthalene with a secondary alcohol at C1, a phenolic alcohol at C8, and a primary alcohol in a −CH2OH group at C4.
Reaction of alcohols with SOCl2 converts −OH groups to −Cl groups. This reaction is effective for primary and secondary alcohols. Phenolic alcohols do not react with SOCl2 under normal conditions to form aryl chlorides.
In the given starting material, we have a primary alcohol (-CH2OH) and a secondary alcohol (at C1). The phenolic alcohol (at C8) will not react. So, the primary alcohol group at C4 will be converted to −CH2Cl, and the secondary alcohol group at C1 will be converted to −Cl. The phenolic −OH group at C8 will remain unchanged.
The total number of halogen atoms in the major product (A) is x. There are two chlorine atoms in the product. So, x=2.
Now, we need to calculate the degree of unsaturation (y) of the major product (A). The formula for degree of unsaturation (DU) is: DU=Number of rings+Number of π bonds
The major product has two fused rings. So, the number of rings = 2. The major product contains an aromatic ring (C4a-C5-C6-C7-C8-C8a). An aromatic ring has 3 double bonds (3 π bonds). So, the number of π bonds = 3. Degree of unsaturation (y) = Number of rings + Number of π bonds = 2 + 3 = 5.
Alternatively, we can calculate the degree of unsaturation using the molecular formula. Let's determine the molecular formula of the major product (A).
The carbon skeleton is the same as the starting material, which is a derivative of 1,2,3,4-tetrahydronaphthalene with an additional carbon in the −CH2Cl group. The fused ring system has 10 carbons. The −CH2Cl group adds one carbon. So, total carbons c=11.
Let's count the hydrogens in the product. C1 is bonded to Cl, C2, C8a and 1 hydrogen. So, C1 has 1 H. C2 has 2 hydrogens. C3 has 2 hydrogens. C4 is bonded to C3, C4a, and CH2Cl. It has 1 hydrogen. C4a has 0 hydrogens. C5 has 1 hydrogen. C6 has 1 hydrogen. C7 has 1 hydrogen. C8 is bonded to C7, C8a, and OH. It has 0 hydrogens. The OH group has 1 hydrogen. C8a has 0 hydrogens. The −CH2Cl group has 2 hydrogens. Total hydrogens h=1+2+2+1+0+1+1+1+0+0+1(from OH)+2(from CH2Cl)=12. Number of nitrogens n=0. Number of oxygens o=1 (from the phenolic OH group). Number of halogens x=2 (from the two Cl atoms). Molecular formula of (A) is C11H12O1Cl2.
Degree of unsaturation DU=22c+2+n−h−x DU=22(11)+2+0−12−2=222+2−12−2=224−14=210=5. So, the degree of unsaturation of (A) is y=5.
We need to find (x+y). x+y=2+5=7.
