Question
Question: Consider the following reaction If A,B,C and D are the four structural isomers of compound Q and ...
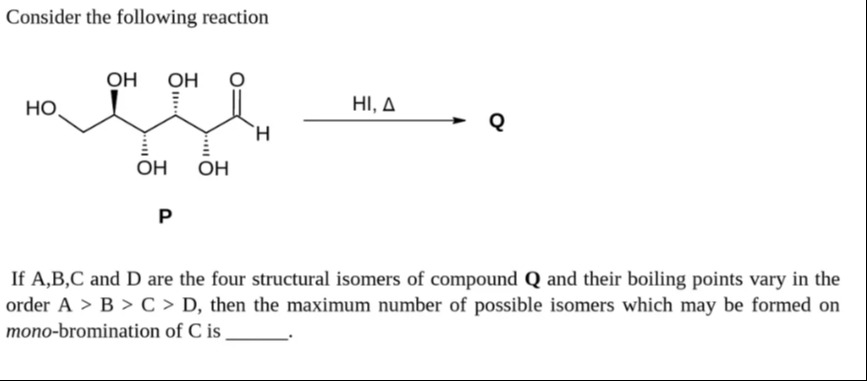
Consider the following reaction
If A,B,C and D are the four structural isomers of compound Q and their boiling points vary in the order A > B > C > D, then the maximum number of possible isomers which may be formed on mono-bromination of C is ____.
7
Solution
The given reaction is of compound P with HI and heat to form compound Q. Compound P is glucose, a six-carbon aldohexose. Reaction of glucose with HI and heat reduces all the oxygen functional groups, leading to the formation of n-hexane. Thus, compound Q is n-hexane (C6H14).
The problem states that A, B, C, and D are four structural isomers of compound Q and their boiling points vary in the order A > B > C > D. The structural isomers of hexane (C6H14) are:
- n-hexane (linear chain)
- 2-methylpentane
- 3-methylpentane
- 2,2-dimethylbutane
- 2,3-dimethylbutane
The boiling points of these isomers are:
- n-hexane: 68.7 °C
- 3-methylpentane: 63.3 °C
- 2-methylpentane: 60.3 °C
- 2,3-dimethylbutane: 58.0 °C
- 2,2-dimethylbutane: 49.7 °C
Based on the boiling point order A > B > C > D, we can assign the isomers:
A = n-hexane (68.7 °C) B = 3-methylpentane (63.3 °C) C = 2-methylpentane (60.3 °C) D = 2,3-dimethylbutane (58.0 °C)
These are four of the five structural isomers of hexane, ordered by decreasing boiling point.
The question asks for the maximum number of possible isomers which may be formed on mono-bromination of C. Compound C is 2-methylpentane. The structure of 2-methylpentane is: CH3 - CH(CH3) - CH2 - CH2 - CH3
We need to find the number of possible mono-brominated products, considering both structural and stereoisomers. Mono-bromination involves replacing one hydrogen atom with a bromine atom. We need to identify the distinct types of hydrogen atoms in 2-methylpentane.
Let's number the carbons in 2-methylpentane using IUPAC numbering:
1 2 3 4 5
CH3 - CH - CH2 - CH2 - CH3 | CH3 6
Possible positions for mono-bromination are on carbons 1, 2, 3, 4, 5, and 6.
- Substitution at C1 (methyl group): Gives CH2Br - CH(CH3) - CH2 - CH2 - CH3. This is 1-bromo-2-methylpentane. The carbon at position 2 is a chiral center (attached to H, CH3, CH2Br, and -CH2CH2CH3). So, this product exists as a pair of enantiomers (R and S). Number of isomers = 2.
- Substitution at C2 (tertiary carbon): Gives CH3 - CBr(CH3) - CH2 - CH2 - CH3. This is 2-bromo-2-methylpentane. There are no chiral centers. Number of isomers = 1.
- Substitution at C3 (secondary carbon): Gives CH3 - CH(CH3) - CHBr - CH2 - CH3. This is 3-bromo-2-methylpentane. The carbon at position 3 is a chiral center (attached to H, Br, -CH(CH3)2, and -CH2CH3). So, this product exists as a pair of enantiomers (R and S). Number of isomers = 2.
- Substitution at C4 (secondary carbon): Gives CH3 - CH(CH3) - CH2 - CHBr - CH3. This is 4-bromo-2-methylpentane. The carbon at position 4 is a chiral center (attached to H, Br, -CH2CH(CH3)2, and -CH3). So, this product exists as a pair of enantiomers (R and S). Number of isomers = 2.
- Substitution at C5 (methyl group): Gives CH3 - CH(CH3) - CH2 - CH2 - CH2Br. This is 1-bromo-2-methylpentane. Numbering from the other end gives 5-bromo-4-methylpentane, which is the same as 1-bromo-2-methylpentane. The carbon at position 2 (from the original numbering) is a chiral center. This product is the same as the one obtained by substitution at C1. Number of isomers from this position is already counted in case 1.
- Substitution at C6 (methyl group attached to C2): Gives CH3 - CH(CH2Br) - CH2 - CH2 - CH3. This is 1-bromo-2-methylpentane. The carbon at position 2 is a chiral center. This product is the same as the one obtained by substitution at C1 and C5. Number of isomers from this position is already counted in case 1.
So, the distinct structural isomers formed by mono-bromination are 1-bromo-2-methylpentane, 2-bromo-2-methylpentane, 3-bromo-2-methylpentane, and 4-bromo-2-methylpentane.
Let's re-examine the positions of substitution and the resulting stereoisomers.
Position 1 (C1): 1-bromo-2-methylpentane. Chiral center at C2. 2 stereoisomers. Position 2 (C2): 2-bromo-2-methylpentane. No chiral centers. 1 stereoisomer. Position 3 (C3): 3-bromo-2-methylpentane. Chiral center at C3. 2 stereoisomers. Position 4 (C4): 4-bromo-2-methylpentane. Chiral center at C4. 2 stereoisomers. Position 5 (C5): Same as position 1. Position 6 (C6): Same as position 1.
However, we need to be careful about whether substitution at different positions leads to the same structural isomer. We have already named the products and confirmed they are distinct structural isomers.
Now let's double-check the chiral centers.
1-bromo-2-methylpentane: CH2Br-CH(CH3)-CH2CH2CH3. Chiral center at C2. 2-bromo-2-methylpentane: CH3-CBr(CH3)-CH2CH2CH3. No chiral centers. 3-bromo-2-methylpentane: CH3-CH(CH3)-CHBr-CH2CH3. Chiral center at C3. 4-bromo-2-methylpentane: CH3-CH(CH3)-CH2-CHBr-CH3. Chiral center at C4.
So, we have 4 distinct structural isomers.
1-bromo-2-methylpentane has 2 stereoisomers (enantiomers). 2-bromo-2-methylpentane has 1 stereoisomer (achiral). 3-bromo-2-methylpentane has 2 stereoisomers (enantiomers). 4-bromo-2-methylpentane has 2 stereoisomers (enantiomers).
The total number of possible isomers (including stereoisomers) is the sum of the number of stereoisomers for each distinct structural isomer.
Total isomers = 2 (from 1-bromo-2-methylpentane) + 1 (from 2-bromo-2-methylpentane) + 2 (from 3-bromo-2-methylpentane) + 2 (from 4-bromo-2-methylpentane) = 7.
Thus, the maximum number of possible isomers which may be formed on mono-bromination of C (2-methylpentane) is 7.
The final answer is 7.
