Question
Question: Consider the following reaction: (7.8 g) (60% yield) What is the mass of the organic product 'P' f...
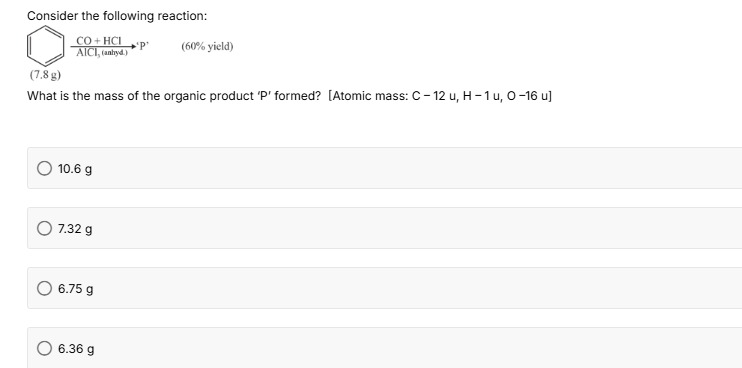
Consider the following reaction:
(7.8 g) (60% yield)
What is the mass of the organic product 'P' formed? [Atomic mass: C - 12 u, H - 1 u, O -16 u]
A
10.6 g
B
7.32 g
C
6.75 g
D
6.36 g
Answer
6.36 g
Explanation
Solution
- Identify the reaction: The reaction of benzene with carbon monoxide (CO) and hydrogen chloride (HCl) in the presence of anhydrous aluminum chloride (AlCl3) is the Gattermann-Koch reaction, which produces benzaldehyde.
- Calculate moles of benzene:
- Molar mass of benzene (C6H6) = (6 × 12) + (6 × 1) = 78 g/mol.
- Given mass of benzene = 7.8 g.
- Moles of benzene = Mass / Molar mass = 7.8 g / 78 g/mol = 0.1 mol.
- Determine theoretical yield of benzaldehyde:
- The reaction stoichiometry between benzene and benzaldehyde (C6H5CHO) is 1:1. Therefore, 0.1 mol of benzene will theoretically produce 0.1 mol of benzaldehyde.
- Molar mass of benzaldehyde (C7H6O) = (7 × 12) + (6 × 1) + (1 × 16) = 84 + 6 + 16 = 106 g/mol.
- Theoretical yield of benzaldehyde = Moles × Molar mass = 0.1 mol × 106 g/mol = 10.6 g.
- Calculate actual yield:
- The reaction yield is given as 60%.
- Actual yield = Theoretical yield × (Percentage yield / 100)
- Actual yield = 10.6 g × (60 / 100) = 10.6 g × 0.6 = 6.36 g.
