Question
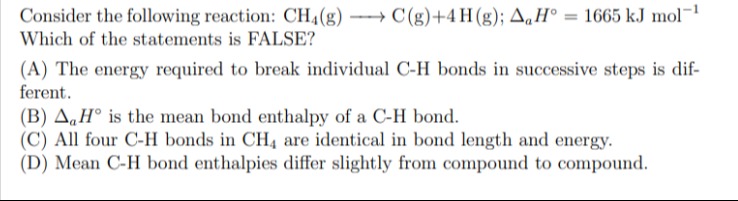
Question: Consider the following reaction: CH4(g) → C(g)+4H (g); ∆aH° = 1665 kJ mol-¹ Which of the statements ...
Consider the following reaction: CH4(g) → C(g)+4H (g); ∆aH° = 1665 kJ mol-¹ Which of the statements is FALSE?
The energy required to break individual C-H bonds in successive steps is different.
∆aH° is the mean bond enthalpy of a C-H bond.
All four C-H bonds in CH4 are identical in bond length and energy.
Mean C-H bond enthalpies differ slightly from compound to compound.
∆aH° is the mean bond enthalpy of a C-H bond.
Solution
The given reaction is the atomization of methane:
CH4(g) → C(g) + 4H(g); ∆aH° = 1665 kJ mol⁻¹
∆aH° is the atomization enthalpy, which is the total energy required to break all the bonds in one mole of gaseous methane to form gaseous atoms. In methane, there are four C-H bonds.
-
Statement A is TRUE: The energy required to break individual C-H bonds in successive steps is different because the chemical environment changes after each hydrogen atom is removed.
-
Statement B is FALSE: ∆aH° is the total energy required to break all four C-H bonds, not the mean bond enthalpy of a C-H bond. The mean bond enthalpy would be ∆aH° / 4.
-
Statement C is TRUE: All four C-H bonds in CH4 are identical in bond length and energy due to the tetrahedral structure of methane.
-
Statement D is TRUE: Mean C-H bond enthalpies differ slightly from compound to compound because the electronic environment around the C-H bond is influenced by the rest of the molecule.
Therefore, the false statement is B.
