Question
Question: Consider the following reaction and select the best choice that represents the reaction. 
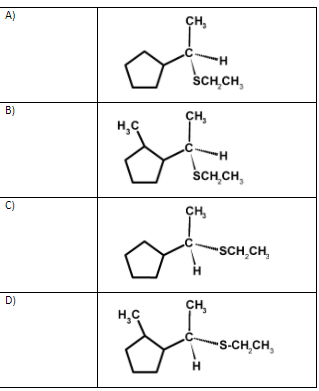
Solution
The nucleophilic substitution reaction of alkyl halides takes place via two pathways: S12 or SN2 . Substitution bimolecular nucleophilic SN2 reaction, the incoming nucleophile attacks on the partially positively charged carbon atom bearing a halogen atom. The reaction takes place via transition state. The product obtained has an inversion of configuration.
Complete step by step answer:
A nucleophilic substitution reaction in which the rate determining steps involves 2 components .i.e. the reaction is bimolecular which contains the simultaneously bond breaking and bond making is known as the SN2 reaction.
Here, the strong nucleophile attacks on the partially positive charged carbon atom of the carbon halogen bond. the nucleophile attack in the direction which is 1800 away from the leaving group.
The sodium ethyl sulphide NaSCH2CH3 is a nucleophile. the nucleophile CH3CH2S− attacks on the partially positive charge carbon atom of a carbon bromine bond C−Br . The carbon has a δ+ charge and the bromine acquires a partial δ− charge. The nucleophile attacks on the carbon from the direction which is 1800 away from the bromine atoms i.e. from the backside. This leads to the partially bond formation between carbon and CH3CH2S− as C........ SCH2CH3 and partially bond breaking between carbon and bromine C........ Br .
This is a one-step reaction.in the transition state, the negative charge is shared by incoming nucleophiles as well as outgoing bromide.
The transition state is unstable because the carbon atom is simultaneously bonded to five atoms and therefore the bromide ion leaves and forming a C−SCH2CH3 bond. The reaction is as shown below,
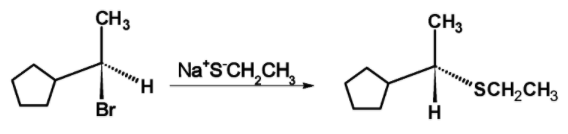
The mechanism of the nucleophilic substitution reaction is shown below,
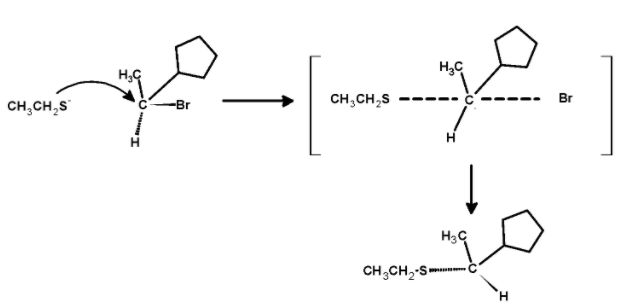
The sulphide nucleophilic backside attack on the carbon atom. Such that the bond which was initially towards the observer now changes to the bond away from the observer. Thus, the molecule undergoes the inversion of configuration.so the product is,
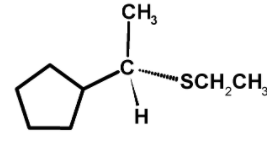
Hence, (C) is the correct option.
Note: Note that, in this reaction bond breaking and making occurs at the same time thus this is also known as the ‘concerted ‘mechanism. The nucleophilic reaction depends on the strength of a nucleophile. The ease at which the nucleophile is displaced or it’s leaving group ability depends on the capacity to accommodate a negative charge. Thus, here bromide leaves the molecule and inverts the configuration.
